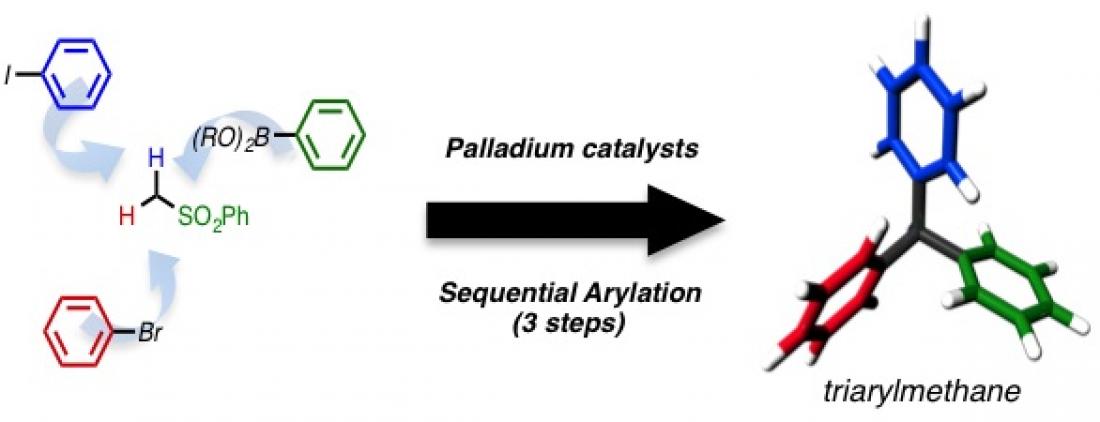
With the Nambo-Crudden group’s synthetic reaction using a palladium catalyst, triarylmethanes can be synthesized in only 3 steps.
Arising from a collaboration between plant and animal biologists, and organic chemists at ITbM, Nagoya University, the group succeeded in developing a new compound, a triarylmethane that can rapidly inhibit cell division in plants. This compound was found to selectively inhibit cell division in plant cells with respect to animal cells. Having a reversible cell inhibiting property, this triarylmethane could be a potential candidate for developing new agrochemicals that can control plant growth.
Nagoya, Japan – Dr. Minako Ueda, Dr. Masakazu Nambo of the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University and their colleagues have reported in the journal Plant and Cell Physiology, on the development of a series of triarylmethane compounds, which were tested on plant cells to see their effect on cell division. Through live cell imaging, they were able to identify a new triarylmethane compound that can rapidly inhibit cell division in plant cells.
They also found that this new compound does not have an effect on the cell division of animal cells, and that cell division restarts in plant cells upon removal of the compound. Being able to control the cell division in plant cells may be effective in controlling plant growth. Thus, the selectivity and reversibility of this new triarylmethane compound on the cell division of plant cells makes it a good candidate for an agrochemical.
Plant growth occurs by increasing the number of cells by cell division followed by enlargement of the cells. Thus, it has been considered that if there is a way to control cell division in plants, this will lead to the control of plant growth in a range of plant species. Although various compounds that can control cell division in plants have been explored in the past, they have mainly resulted in damage to the plant shape or irreversible inhibition of cell division despite removal of the compounds.
“As part of ITbM’s interdisciplinary research initiative, we decided to search for new compounds that can inhibit the cell division in plants without causing damage to them,” says Minako Ueda, a plant biologist and a leader of this study. “Being in the Mix Lab (special labs that have researchers from different disciplines mixed together) at ITbM, I was able to talk to an organic chemist, Masakazu Nambo, who suggested the use of triarylmethane compounds for cell division inhibition in plant cells,” she continues.
“We had reported a new catalytic reaction in December 2013, to rapidly synthesize triarylmethanes in 3 steps from readily available starting materials, using a palladium catalyst,” says Masakazu Nambo, an organic chemist and another leader of this study. “Triarylmethanes have not really been used before on plants, but we were able to visualize their effect on Tobacco plant cells using live cell imaging. We started this research about 3 years ago, but we were fortunate to be able to identify a triarylmethane compound that can rapidly inhibit cell division in plants,” he continues.
Triarylmethanes are a group of compounds that derive from methane (a molecule consisting of carbon with 4 hydrogen atoms attached to it) and consist of a carbon atom center with 3 aryl (aromatic ring) groups and a hydrogen atom. This structure can be found in organic materials, such as dyes and fluorescent probes, as well as in natural products. Some compounds containing the triarylmethane moiety are known to exhibit anticancer properties, and many new compounds have been synthesized to investigate their bioactivities.
“Our palladium-catalyzed sequential arylation reaction has been highly useful to rapidly synthesize a variety of triarylmethanes to be used for testing their effect on the cell division in plants,” says Nambo.
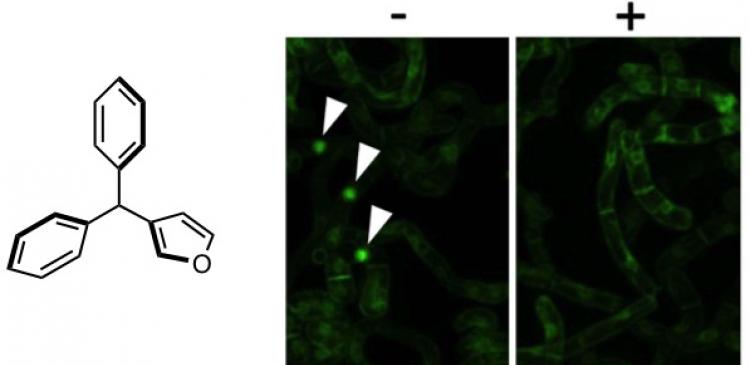
“We used a Tobacco plant cell attached with a fluorescent probe to visualize the cell division process,” says Ueda. “We added the triarylmethane compounds to the cultivated cells and investigated whether cell division had occurred or not by realtime live cell imaging.”
“As a result of screening about 200 compounds, we found that (3-furyl)diphenylmethane (chem7), which is a triarylmethane that contains 2 phenyl groups and a furyl (a 5-membered aromatic ring containing 4 carbons and an oxygen atom in the ring) group, had strong inhibitory activity on plant cell division,” says Ueda.
When the furyl moiety was replaced with other aromatic groups, or when one of the benzene rings was removed, the cell division inhibitory activity was not observed, suggesting that a triarylmethane structure containing both the benzene and the furan rings are necessary for their bioactivity.
“Although I did not have any issues about working with compounds directly synthesized by chemists, I was initially surprised to receive compounds that were not necessarily soluble in the solvents that I was using in my biological experiments,” says Ueda. “It was exciting to test new compounds and I was astonished by the speed that the compounds were being synthesized. The speed of compounds being generated was faster than the speed that we could test them on the cells.”
The group also tested whether chem7 could inhibit cell division in other plants, or in other developing tissues. By applying chem7 to the young seeds and roots of a model plant, Arabidopsis thaliana, the group found that rapid inhibition of cell division was observed in both tissues.
“We saw that chem7 had hardly any effect on the shapes of the cells and tissues, thus, suggesting that chem7 stops cell division in plant cells, but does not cause any severe damage to the shapes,” describes Ueda. “With the help of animal biologists, we found that chem7 had no effect on budding yeasts and human cells, which indicates that chem7 does not inhibit the cell division of animal cells.”
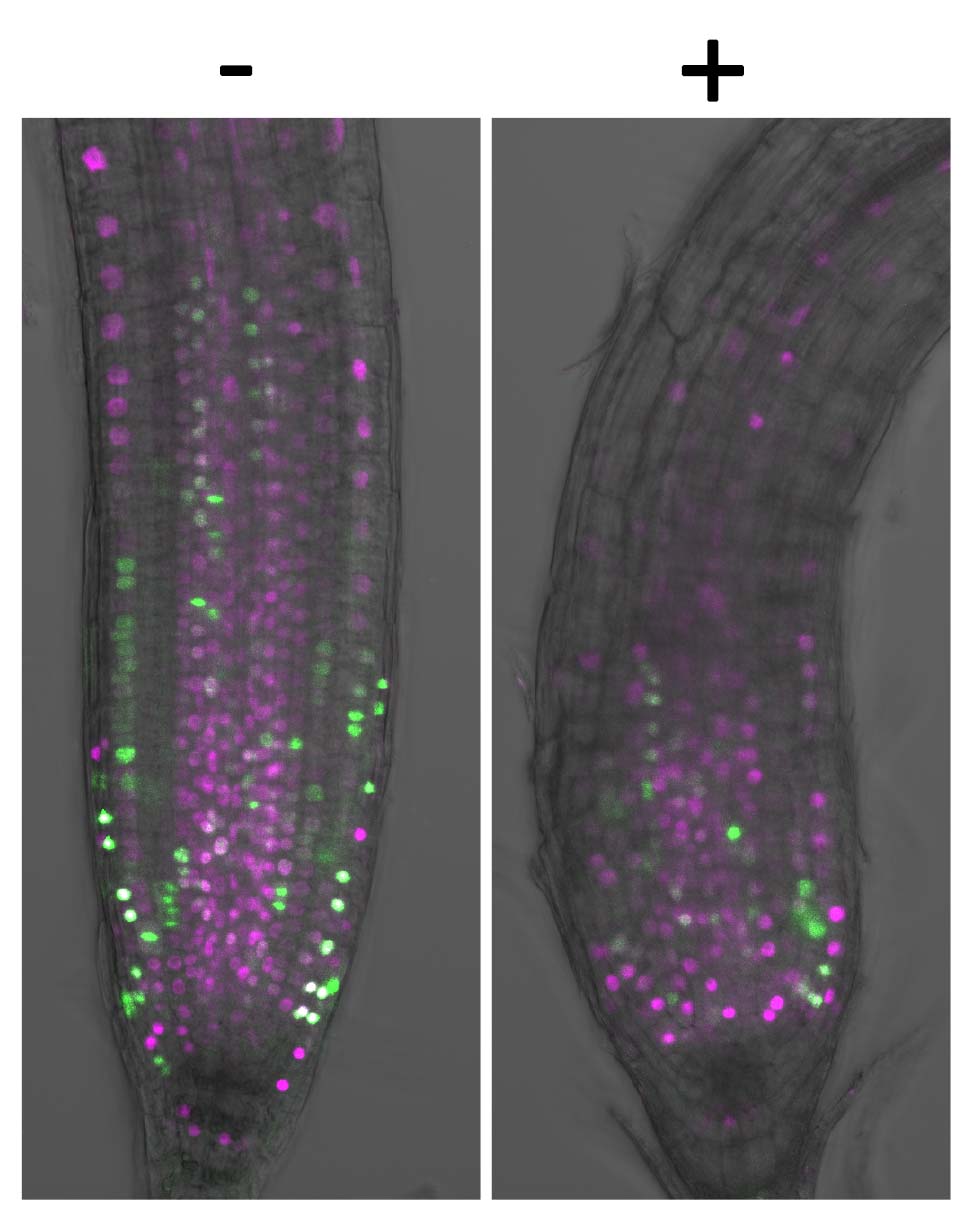
Cell division consists of several phases, including the M phase where the cells actually divide (M = mitosis), the S phase where the DNA is copied and prepares for division (S = synthesis) and the G1/G2 phase in between (G = gap). These phases (cell cycle) are repeated leading to cell division. In order to figure out which phase that chem7 actually acts upon, Ueda and her team used two fluorescent proteins of different colors to visualize the process of the cell cycles in the root of Arabidopsis thaliana. (Green indicates the M phase and red indicates the S and G2 phases.)
“As the roots of Arabidopsis thaliana contain cells at various phases, it was possible to observe different phases, shown in green and red,” explains Ueda. “Upon addition of chem7 to the roots, we found that both colors existed but the area that contains fluoresced cells (tissues with high cell division activity) became smaller.”
This indicates that chem7 does not target a specific plant cell phase, but exhibits cell inhibitory activity regardless of the phase. The group concluded that chem7 causes no severe damage to the shapes of cells and tissues by being able to rapidly stop the cell activity at any cell phase.
In addition, when chem7 was washed away from the roots and cultivated cells treated with chem7, cell division was observed again, indicating that the effect of chem7 is not lethal.
“Through the collaboration with chemists and biologists, we were fortunate to discover a new compound that can selectively inhibit the cell division of plant cells regardless of the cell phase,” says Ueda and Nambo. “chem7 rapidly stops cell division and plant growth without causing drastic damage to the shapes or functions of the cells.”
“It was nice to be able to come together and discuss research with people from different research fields. We are currently carrying out further studies to generate new compounds that can rapidly and reversibly control plant growth without causing harm to humans and bacteria in the surrounding environment, which can potentially work as agrochemicals,” they speak.
This article “Combination of Synthetic Chemistry and Live-Cell Imaging Identified a Rapid Cell Division Inhibitor in Tobacco and Arabidopsis thaliana” by Masakazu Nambo, Daisuke Kurihara, Tomomi Yamada, Taeko Nishiwaki-Ohkawa, Naoya Kadofusa, Yusuke Kimata, Keiko Kuwata, Masaaki Umeda and Minako Ueda is published online in Plant and Cell Physiology. DOI: 10.1093/pcp/pcw140 (http://dx.doi.org/10.1093/pcp/pcw140)
About WPI-ITbM (http://www.itbm.nagoya-u.ac.jp/)
The Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. ITbM is one of the research centers of the Japanese MEXT (Ministry of Education, Culture, Sports, Science and Technology) program, the World Premier International Research Center Initiative (WPI). The aim of ITbM is to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a "Mix-Lab" style, where international young researchers from various fields work together side-by-side in the same lab, enabling interdisciplinary interaction. Through these endeavors, ITbM will create "transformative bio-molecules" that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
Author Contact
Designated Lecturer Minako Ueda
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-747-6402
E-mail: [email protected]
Assistant Professor Masakazu Nambo
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-788-6245
E-mail: [email protected]
Media Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3053
E-mail: [email protected]
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
E-mail: [email protected]