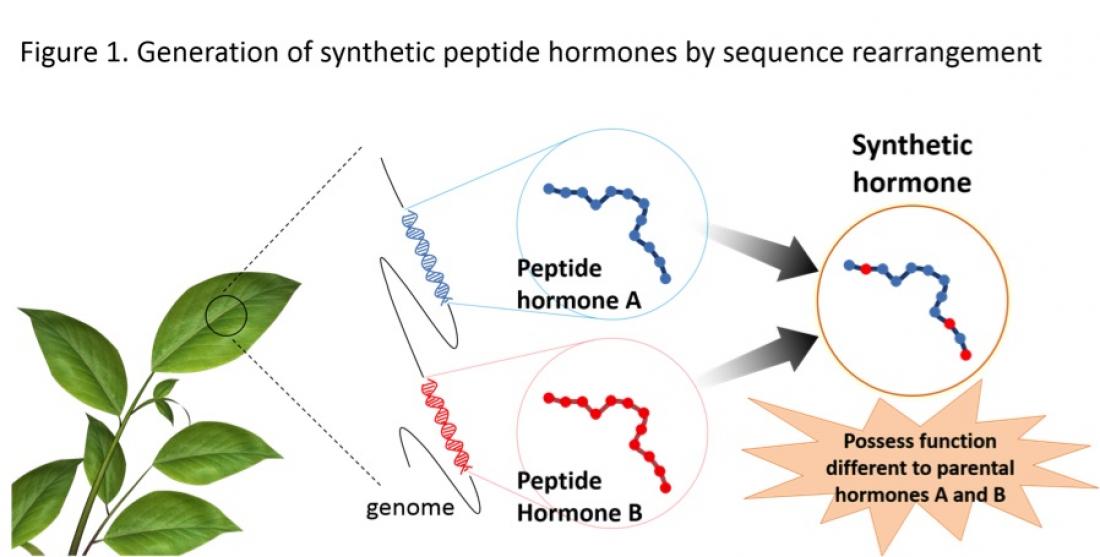
Systematic rearrangement leads to a specific amino acid sequence that enables the peptide to exhibit two functions, instead of just one. This approach is expected to lead to the generation of novel synthetic plant peptide hormones, which can have multiple functions to effectively control plant growth.
Researchers at the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University and their collaborators, have reported in the journal Nature Communications, on their new approach to generate synthetic plant peptide hormones in the model plant Arabidobsis thaliana, by systematic rearrangement of amino acids. The team succeeded in adding the functions of two peptides into one, which opens doors to generating artificial peptide hormones with multiple functions.
A variety of peptide hormones are present in plants, and each peptide is known to have a specific role in plant growth, e.g. determining the shapes of flowers and leaves, controlling the length of roots, and thickening of stems. Peptide hormones consist of approximately 5-100 amino acids, and each hormone has a specific amino acid sequence, which is defined by the genome information.
By rearranging the amino acid sequence of these naturally occurring plant peptides, Yuki Hirakawa, a plant biologist who is a postdoctoral researcher at ITbM, made an artificial peptide that can trigger more than one function.
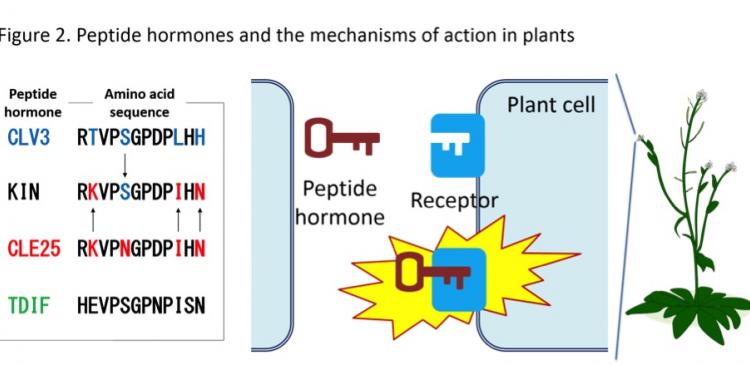
The group focused on the two plant peptides, CLV3 and CLE25, which control the growth of roots. Both peptides belong to the CLE (CLAVATA3/EMBRYO SURROUNDING REGION-related) family and have slightly different sequences. By swapping the sequences of the two peptides, the group succeeded in generating a synthetic peptide KIN that exhibits vascular-thickening function on top of the original root-shortening function. Vascular tissues are responsible for transporting water and nutrients throughout the plant.
"We were really surprised to see that the KIN peptide exhibited new functions, which are different to the CLV3 and CLE25 peptides," explains Hirakawa. KIN stands for the single letter codes of the three amino acid residues that were swapped from the CLV3 peptide (Fig. 2; K = lysine, I = isoleucine, N = asparagine).
"Peptide hormones work in a "lock and key" mechanism, where the key (peptide hormone) specifically binds to a particular lock (protein receptor), which is located on the surface of the plant cell," says Naoyuki Uchida, a plant biologist and an associate professor at ITbM. "Once the lock accepts a key, the cell is stimulated and eventually affects the growth of plants," he continues.
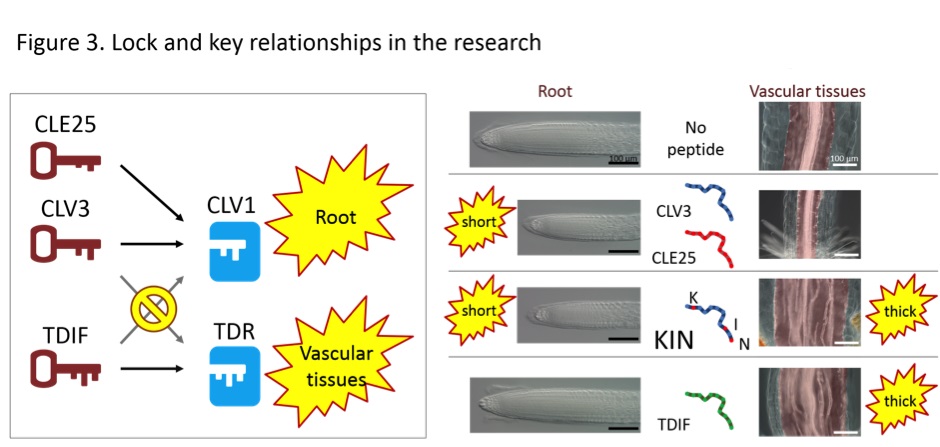
CLV3 and CLE25 peptides are able to act on the CLV1 receptor. Since the synthetic peptide has functions different from the parental peptides, the group hypothesized that the synthetic peptide now acts on a receptor different from CLV1.
A good candidate for this additional receptor was TDR (TDIF receptor) because its key, TDIF (tracheary element differentiation inhibitory factor) peptide, functions in vascular-thickening. It is worth noting that CLE25 and CLV3 peptides are unable to act on the TDR receptor and vice versa (Fig. 3).
The group performed biochemical experiments and confirmed that the newly synthesized KIN peptide is able to bind to both receptors, CLV1 and TDR. In other words, the KIN peptide is a key that acts on two different locks.
Computer simulations conducted by Kai Welke, a theoretical chemist who is a postdoctoral researcher and Stephan Irle, also a theoretical chemist and professor at ITbM, analyzed the contribution of amino acid in peptides in the atomic-scale dynamics of interaction with TDR, which allowed further experimental examination of the peptide-protein interactions.
"Our studies show that we're able to change the lock and key relationship in receptors and peptide hormones, through partial rearrangement of the amino acid sequence in plant hormones," says Keiko Torii, a plant biologist and a professor at ITbM and the University of Washington.
The group says that their approach makes use of the diverse genetic network of plant peptide hormones, which can generate numerous combinations of peptides. They hope that their method can be applied for the development of other plant peptide hormones, which will be useful for generating crops with improved properties.