Subtypes of HPV that infect the genital tract.
Introduction
‘For me, an area of moral clarity is: you’re in front of someone who’s suffering and you have the tools at your disposal to alleviate that suffering or even eradicate it, and you act’ Dr.Paul Farmer (American Anthropologist and Physician)
Cervical cancer is a preventable disease and yet globally, more than half a million women, who are at the prime of their lives, are diagnosed with it every year. In Malaysia, at least 6 women are diagnosed with predominantly advanced disease every day. This situation is largely preventable because unlike other cancers, this is a cancer where effective screening tools and preventative vaccines are readily and widely available in Malaysia. In recent years, advances in the approach towards screening for cervical cancer and the availability of HPV vaccines means that cervical cancer should no longer be robbing our women prematurely of their health.
What is the cause of cervical cancer?
Cervical cancer is unique as it is the only cancer where an infective agent is required for its development. In 1999, the World Health Organization classified human papillomavirus (HPV) as a necessary cause of cervical cancer. While there are other factors that contribute to its development such as cigarette smoking and weakened immune system, persistent infection with oncogenic (cancer causing) types of HPV is attributable to the development of cancer.
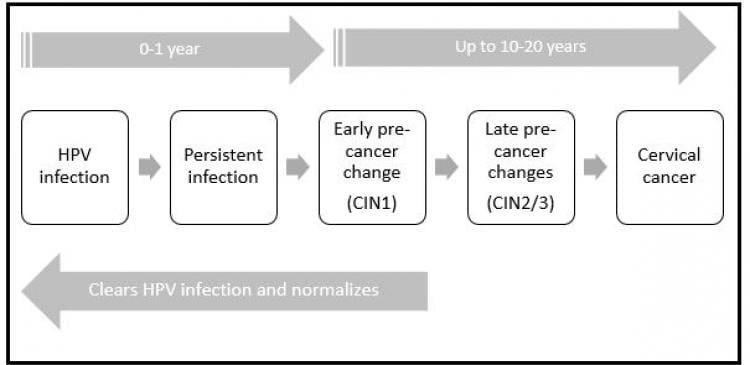
There are more than 200 types of HPV known with 40 types infecting the genital tract. HPV is also responsible for the development of the common wart and genital warts. The subtypes that cause warts (low risk HPV) do not cause cancer. There are 14 subtypes of HPV ( Type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) that have been classified as being oncogenic (high risk). See Figure 1 This means that it has the ability to cause cancer in a minority of individuals. Globally, HPV 16 and 18 are responsible for causing 70% of cervical cancer while Types 16, 18, 31, 33, 45, 52 and 58 cumulatively account for 90% of all cervical cancers.
How is HPV acquired and how can it be treated?
Infection with HPV is extremely common with 80% of women having a transient infection in their lifetime. Infection with HPV is generally transient and goes unnoticed most of the time. The most common mode of transmission is by sexual contact. The acquisition of HPV usually occurs at sexual debut. However, transmission of infection does not translate to transmission of disease. Even babies born naturally from mothers who are infected with HPV can have HPV detected in their oral cavities transiently (vertical transmission). Most individuals infected with HPV clear the infection spontaneously (within a year) but in a minority, the infection persists for years and may lead to cervical cancer in women. See figure 1. The control of HPV infection is by our immune system. Therefore, in situations where a patient is immune-compromised, such as transplant patients and people living with HIV, these individuals are more susceptible to persistent HPV infection.
How can cervical cancer be avoided?
While there is no antibiotics for the treatment of HPV infection, there are ways to detect the presence of HR HPV infection and abnormal changes to the cervix. From observational studies, it is estimated that cervical cancer generally takes approximately 10 years to develop. The slow progress of disease allows of opportunities for detection through ‘screening’. This is the basis of cervical cancer screening. When a woman has a pap smear, cells are taken from the cervix and sent to the laboratory for interpretation. However, there are occasions where the interpretation of the cells are difficult which can result in a women requiring a repeat pap smear or a ‘false’ result being generated. These are some of the disadvantages of the cervical cancer screening program.
However, it must be emphasized that cervical cancer screening saves lives. In countries such as the United Kingdom, Australia, Netherlands and Sweden where organized screening programs are in place, the incidence and mortality from cervical cancer have decreased significantly. Unfortunately, in Malaysia, only 1 in 5 women who should have a Pap smear will ever have one. The current cervical cancer screening recommends that women aged 25-60 sees a healthcare professional to have a pelvic examination and pap smear taken every 3 years. This translates to approximately 15 pap smears in a woman’s lifetime provided all the tests are reported as normal. However, the majority of women do not adhere to the recommended screening schedule. This is despite the regular campaigns and relatively easy access to healthcare facilities. The reasons commonly quoted for the poor uptake of regular Pap smears include embarrassment, discomfort, fear of pelvic examinations, lack of time, feeling they are unnecessary and not relevant to them.
What about HPV vaccines? Who? When? Safety?
Since HR HPV is a prerequisite for the development of cervical cancer, preventing persistent HR HPV infection should remove the risk of cervical cancer. This is the basis of HPV vaccination. As mentioned earlier, 70% of all cervical cancers are due to HPV 16 and HPV 18 while types 16, 18, 31, 33, 45, 52 and 58 are cumulatively responsible for 90%. Hypothetically, this means that if a population is protected against HPV 16 and 18, there will be a 70% reduction of cervical cancer while if they are protected from the additional 5 types, there will be a corresponding reduction of 90% in the incidence of cervical cancer.
The Food and Drug Administration (FDA) approved the first HPV vaccines in 2006 after extensive testing and clinical trials. The initial 2 HPV vaccines were designed mainly to protect women from HPV 16 and 18 associated cancers and pre cancers (in addition, one of them also protected women against genital warts). The clinical trials looked at many different outcomes. However, the most important findings were that the vaccines were safe and highly effective in preventing precancerous changes of the cervix resulting from the vaccine-specific HPV subtype. For example, the bivalent vaccine (Cervarix©) protected women against HPV 16 and 18 associated cancers while the quadrivalent vaccine (Gardasil©) protected women against HPV 16 and 18 associated cancers and genital warts secondary to HPV 6 and 11. For the vaccines to be most effective, it should ideally be given to girls prior to HPV exposure i.e. sexual debut. This is why in countries such as Malaysia where a national vaccination program exists; the vaccines are administered at the age of 11-13 years old. These highly effective vaccines have been approved in more than 150 countries with government funded national immunization programs existing in 70-80 countries. The safety of the vaccines are being continuously monitored and the World Health Organization’s Global advisory committee on vaccine safety.
Developments in the area of cervical cancer prevention
(a) Secondary prevention by cervical cancer screening
In recent years, many large studies have demonstrated that screening for the presence of HR HPV is more sensitive and effective method for cervical cancer screening compared to the conventional pap smear. The technological improvements enable the presence of HR-HPV to be detected from swabs/samples taken without the need for an uncomfortable pelvic examination. In fact, women can even take the swabs themselves (self-swab). Using improved and evidence-based screening methods which are now available, a woman may only need as few as 3-5 screening tests in their lifetime to prevent cervical cancer. Because we know that persistent infection with a HR HPV is a prerequisite for the development of precancerous changes and cancer of the cervix, a woman who screens negative for HR HPV, can be reassured that she will have minimal risk of developing cervical cancer. In many countries, the policy of using HPV testing is now being adopted as the primary screening tool. In countries that have implemented HPV vaccination, the incidence of abnormal changes to the cervix are already on the decline. In this situation, a more sensitive screening tool, such as HPV DNA testing would be required to identify women who remain at risks of developing cervical cancer. In addition, many other molecular tests that can identify precancerous changes to the cervix more accurately are being developed and tested.
What is important when considering implementing new cervical screening strategies is the acceptability, scalability and sustainability unique to the population’s socio-demographics. Central to a successful screening program is one that socio-culturally acceptable with clear management pathways. In the developing world where the infrastructure for the conventional ‘call and recall’ systems for cervical cytology does not exists, point-of care, ‘see and treat’ strategies may be more appropriate. What is critical is to utilize or innovate health-care systems that can be deployed effectively in the developing world.
(b) Primary prevention by HPV vaccination
Since 2006, two HPV vaccines have been marketed and used in the national vaccination programs throughout the world. Both vaccines have been shown to be highly effective against HPV 16 and 18 related precancerous changes of the cervix and therefore cervical cancer. Furthermore, the quadrivalent vaccine is also highly effective in preventing the genital warts particularly in Australia where new incidence of genital warts among women vaccinated women have precipitously plummeted by 92%.
In 2016, the FDA in the USA approved the nine-valent HPV vaccine (Gardasil 9) vaccine which was protective against 5 further subtypes of high risk HPV (31, 33, 45, 52 and 58). This was vaccine has since received regulatory approval in 50 countries (as of Jan 2017) including Malaysia. The clinical trials have demonstrated efficacy against HPV types 6, 11, 16, and 18 to be non-inferior to the current quadrivalent vaccine BUT with additional protection against the other 5 subtypes. This is an exciting development as we now have the potential to prevent 90% of HPV associated cervical cancers by vaccinating the primary target group of young adolescent girls aged 9-14. While the cost of the vaccine is prohibitive, more innovative procurement negotiations can be instituted such as that demonstrated by PATH (www.path.org), an international health organization driving transformative innovation.
(c) Multi-disciplinary approach in cervical cancer prevention
While technologies (point of care screening tools, vaccines) and resources can be made available, drivers for change and adopting of effective screening strategies need to emerge and be ‘owned’ by invested stakeholders of a particular country. Strong political champions and leadership are a prerequisite in ensuring that policies and solutions are enforced and sustained. Developing innovative public private partnerships to maximize life-saving strategies will be important. Practically speaking, this means the getting together of governmental and non-governmental organizations, public and private institutions backed by sufficient financial resources to initiate effective cervical cancer strategies.
Conclusion: Cervical cancer, the second most common cancer among women of reproductive age globally can be prevented. There is no ONE cancer where such effective tools (vaccines and screening) are readily available to be used for it’s prevention. The emphasis now should now be redirected towards implementation of these technologies innovatively in a sustainable fashion.
Prof. Dr. Woo Yin Ling
Department of Obstetrics and Gynaecology
Faculty of Medicine,
University of Malaya, 50603 Kuala Lumpur, Malaysia.
[email protected]