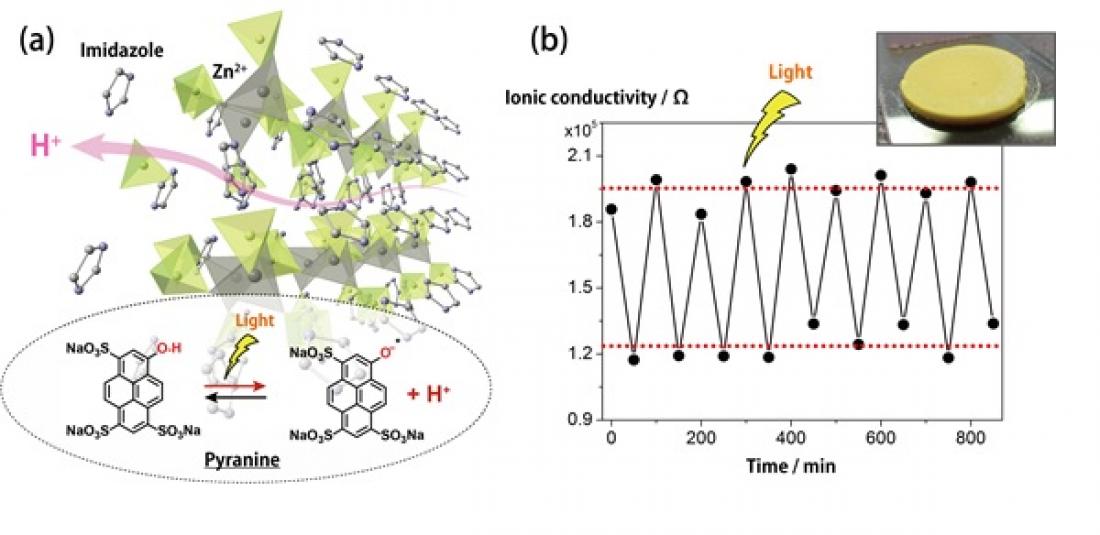
Figure. (a) Crystal structure of H+ conductive coordination polymer and doped pyranine molecule, which releases/captures H+ by light irradiation. (b) Observed H+ conductivity switching property by on/off light irradiation.
Researchers at the Institute for Integrated Cell-Material Sciences (iCeMS) in Japan have demonstrated an on/off switching behaviour in a coordination polymer crystal.
Coordination polymer crystals are inorganic and organic hybrid materials. They are known for their structural and functional diversity and their ability to conduct protons.
Proton conduction is a form of electrical conduction in which positive hydrogen ions (H+) carry the charge instead of electrons. It plays a key role in powering photosynthesis in plants and could be used to develop better fuel cells.
A team of researchers led by Satoshi Horike and Susumu Kitagawa synthesized a coordination polymer (CP) by reacting zinc oxide, phosphoric acid and imidazole in ethyl alcohol at room temperature. The CP was then melted and triflic acid was added. The resultant mixture was then cooled and recrystallized. This ‘acid doping’ of the CP significantly enhanced its proton conductivity.
The team melted their original CP again and instead added the ‘photoacid’ pyranine. Photoacids are molecules that become more acidic upon absorption of light. After cooling the material, its now recrystallized form was exposed to light and its proton conductivity improved. When the light was turned off, its conductivity decreased and returned to its original state. This change could be switched on and off over several consecutive cycles of light exposure.
Acid doping of the CP resulted in minimal structural change with overall enhancement of proton conductivity. Doping the CP with photoacid gave the researchers on-demand external control of the ionic current in the material.
“This is the first demonstration of utilization of the melting state for CP functionalization,” conclude the researchers in their study published in the journal Angewandte Chemie. Their melt-doping strategy could potentially be extended to synthesize a new class of proton-conducting solids that can be used in non-volatile memory technologies, ionics-based transistors, and light-induced ionic/electric current circuits.
The Institute for Integrated Cell-Material Sciences (iCeMS) at Kyoto University in Japan aims to advance the integration of cell and material sciences, both traditionally strong fields at the university, in a uniquely innovative global research environment. iCeMS combines the biosciences, chemistry, materials science and physics to create materials for mesoscopic cell control and cell-inspired materials. Such developments hold promise for significant advances in medicine, pharmaceutical studies, the environment and industry.
For information on the research:
Dr. Satoshi Horike
Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University Institute for Advanced Study
E-mail: [email protected]
For information on iCeMS:
Ms. Izumi Mindy Takamiya, Public Relations Officer
Kyoto University Institute for Advanced Study
Phone: +81-75-753-9755
E-mail: [email protected]