Professor Sangaraju Shanmugam (left) and Ph.D Student Arumugam Sivanantham (right)
A research team led by Professor Sangaraju Shanmugam of Energy Science and Engineering at DGIST has developed highly efficient and ultra-durable core-shell nanostructured electrocatalyst and successfully replaced the precious anode in water electrolysis, through the collaboration with the research group of Pacific Northwest National Laboratory (PNNL), USA.
The replacement of conventional fuels with renewable energy resources is a suitable approach to achieving an eco-friendly environment and decreasing future energy demands. To these ends, electrochemical energy generation or conversion in renewable energy devices, which depends on anode and cathode reactions, has received much attention.
In electrocatalytic water splitting, oxygen gas generates in the anode due to the oxygen evolution reaction (OER), and it is a slow electrochemical reaction as compared with the hydrogen evolution reaction (HER). Thus, a suitable electrocatalyst is needed for promising and stable electrocatalytic water splitting.
Development of efficient, durable, low-cost OER electrocatalysts is a great challenge and paid more attention to the renewable energy devices of the water electrolyzer. Until now, the Ruthenium and Iridium oxides are considered as state-of-the-art electrocatalysts in OER, but the lack of stability limit their use in large-scale water splitting and hinder widespread commercialization.
Hence, Professor Shanmugam’s and PNNL teams has focused on developing an alternative, low-cost, non-noble metal electrocatalyst to replace the noble metal anode electrode in efficient water splitting. Carbon-supported metal has been considered as an efficient electrocatalytic material for the enhanced OER in water splitting. So far, most of the developed electrocatalysts have featured higher carbon content and less metal active specious content. The higher carbon amount mired the real metal active sites and thus resulted in a faster carbon corrosion conditions. This further led to lower electrocatalytic activity, stability and large-scale water splitting processes (scalability).
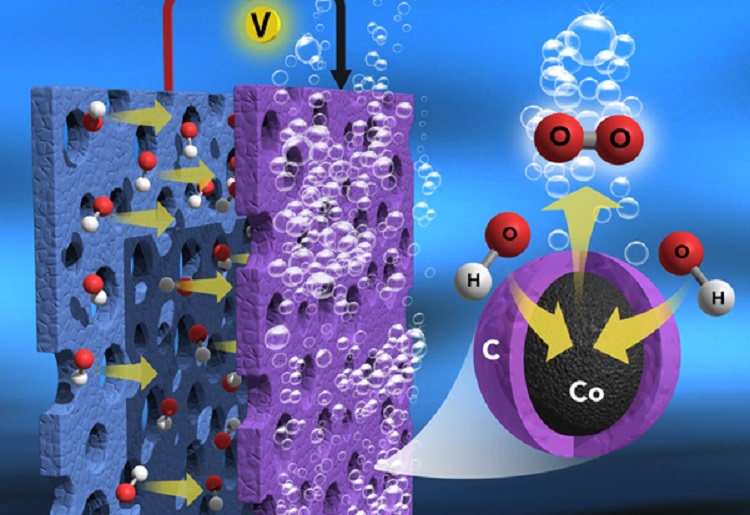
In the study, the researchers found that a large amount of inorganic cobalt metal ions bridged by organic ligands in the Prussian blue analog were shown to be a suitable precursor for developing efficient and ultra-stable, metal-rich, nitrogen-doped graphitic nanocarbon-encapsulated core-shell electrocatalysts for the sluggish OER (anode) in water splitting.
When heated (600–900°C) in an inert atmosphere, the cobalt metal ions and organic ligands in the salt are transformed into cobalt metal and nitrogen-doped graphitic thin carbon layers, respectively, which form the thin carbon layer, encapsulated metallic, cobalt core-shell nanostructures (Core-Shell Co@NC). The thin carbon layers have a strong interaction with cobalt metal, which can promote less carbon corrosion, excellent electron movement, and more cobalt metal exposure to the reaction medium, including the formation of nanosized morphology without particle aggregation.
Accordingly, the combined effect of carbon and cobalt metal in the electrodes achieves the more efficient electrocatalytic activity of the OER than that of the precious metal electrodes to allow efficient water splitting. Therefore, the non-noble metal-rich electrode is an alternative, active, stable, and less expensive OER anode for cost-effective H2 gas production in commercial-scale water electrolysis.
"Anticipate this to be a unique approach to developing metal-rich, reduced-carbon composite nanostructures that have enhanced metal active sites, which feature thin carbon layer protection and ultra-fast electron movement in the catalyst surface, that will enhance the electrochemical activity and stability of electrocatalysts,” says Professor Shanmugam. “We will carry out the follow-up studies that can be used to understand the real OER mechanism on the active species in the presence of nanocarbon coating".
This research outcome was published in the online edition of Advanced Energy Materials on 11th of January 2018, a reputed international journal in the field emerging materials.
The following is an interview with Professor Sangaraju Shanmugam (Department of Energy Science & Engineering):
Q. What are the differences compare to the previous researches?
A. In the previous research, the researchers have prepared the carbon coated metals from various precursors, including metal-organic frameworks (MOFs). The obtained catalysts exhibit more carbon with less graphitic nature, and the carbon covered the active metal sites. Thus most of the active metal sites are not utilized properly by the electrochemical reactions. Also, due to the substantial carbon corrosion, those catalysts are not suitable enough for the sluggish OER in water splitting at the higher positive potential with lack of instability in harsh electrolyte conditions. Accordingly, in this work, we prepared the metal-rich, thin nanocarbon (NC) layers encapsulated electrocatalyst of core-shell Co@NC nanostructures from a single precursor Prussian blue (PB) analogue. The Co@NC showed enhanced oxygen evolution activity and ultrastability on the current collector of nickel foam. Overall, the thin and uniform carbon layers provide the fast electron movements, more metal active sites utilization with easy electrolyte penetration. Most importantly, it can protect the active metal sites from the corrosion with minimal exposing and also the strong interaction between metal and carbon layers exhibits the synergistic effect towards the excellent activity and ultra-stability (over 350 h) of core-shell Co@NC nanostructures with less possibility of carbon oxidation.
Q. How can it be utilized?
A. Based on the remarkable OER performance, kinetics and long-term stability of core-shell Co@NC nanostructures as compared to the state-of-the-art Noble metal based electrocatalysts, such as IrO2 and RuO2, it can be the most suitable candidate to replace the precious OER electrode for reducing the overall cost of the water electrolyzer system. Thus the development of efficient and durable non-noble metal electrocatalyst in water electrolyzer is the main obstacle for successful commercialization of water electrolyzers.
Q. How long will it be required for commercialization?
A. The know-how process is readily available for the fabrication of cost-effective catalyst. But, still we have to evaluate the integration of this catalytic system in a polymer electrolyte membrane electrolyzer, and moreover, studies are underway to understand the OER mechanism on this electrocatalyst and which can help us to realize their activity lose and some other issues in the large-scale water electrolyzer. So, for the commercialization, it may require a year with complete understanding towards activity and stability.
Q. What are the challenges for commercialization?
A. We have to make the uniform coating of this catalyst on the larger size current collectors without any peeling off. So, we need to find the more suitable coating methodology. Also, as like precious OER electrocatalysts we have to understand the precise OER mechanism on this electrocatalysts to maintain/avoid activity losses due to the unwanted side reactions, etc.
Q. Please let us know the motivation on you research.
A. The primary motivation of this research work is that the development of non-precious, metal active sites rich, core-shell electrode material to replace the precious anode in the water electrolyzer system with excellent activity and stability. So, to improve the activity and stability, we tried to introduce the very thin carbon coating on the metal active sites. Overall, the development metal-rich and carbon less OER electrocatalysts with proper utilization of metal-active species and metal-carbon synergistic effect to overcome the sluggish anode reaction in water electrolysis.
Q. What is the final goal you would like to achieve through this research?
A. Based on this research, we understand that the metal-rich electrocatalysts are one of the most suitable materials for the excellent OER activity. So, we want to prepare the cheapest anode electrocatalysts by using the same methodology and want to eliminate the use of precious electrodes in the water electrolyzer system for the production of green and sustainable hydrogen in large scale.
For more information, please contact:
Associate Professor Sangaraju Shanmugam
Department of Energy Science and Engineering
Daegu Gyeongbuk Institute of Science and Technology (DGIST)
E-mail: [email protected]
Oxygen evolution on the surface of core-shell Co@NC electrode in alkaline water electrolysis