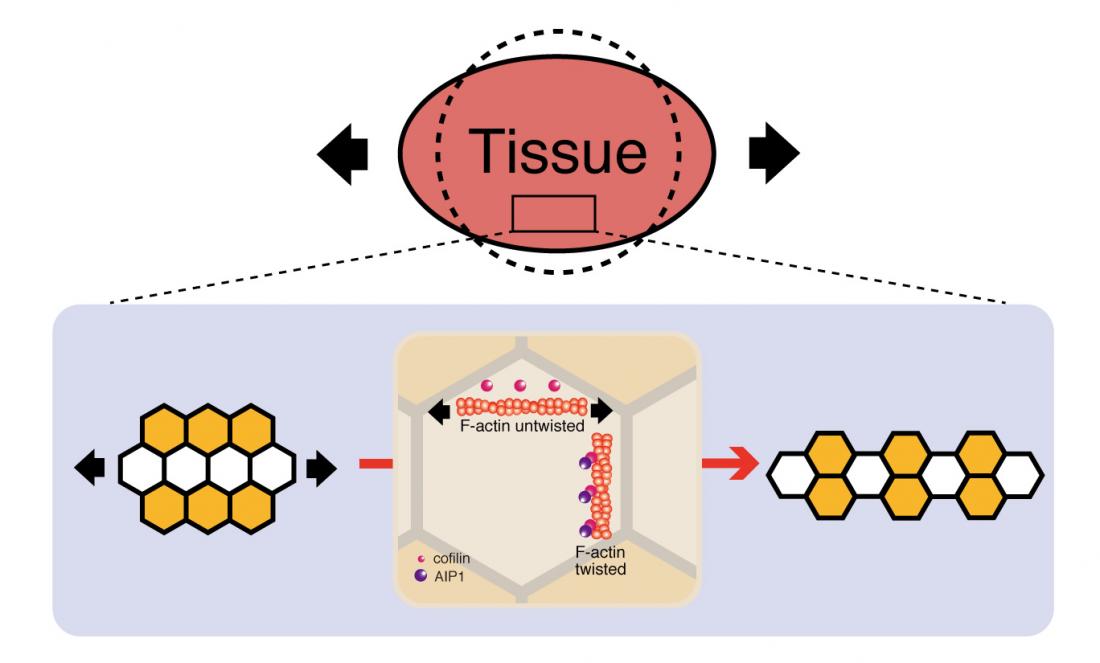
In response to tissue tension, cells change their positions along the direction of tissue stretching. AIP1 and cofilin maintain the structural stability of remodeling junctions and thereby promote directional cell rearrangement.
Research about mechanical control of tissue development is published in Nature Communications this week. The article identifies the protein which regulates cell rearrangement in response to increasing tissue tension. This protein, AIP1, and its cofactor cofilin, could be involved in tissue tension-driven cell rearrangement across species and the activity could apply to cell proliferation and death. During development, cells change their positions along the tissue axis to shape tissue. The global patterns of forces in a tissue, including tension and compression, influence growth. Although knowledge of the molecular mechanisms of force generation inside a cell has grown in recent years, much less is known about how a cell responds to and resist tissue tension when cell contact surfaces are being remodeled. Kaoru Sugimura and Keisuke Ikawa of the Institute for Integrated Cell-Material Sciences (iCeMS) at Kyoto University in Japan used the Drosophila pupal wing in their research. They screened actin binding proteins, and found that Actin binding protein 1 (AIP1) regulates cell arrangement in the wing, with strong signals where cell rearrangement is taking place. They found less AIP1 once the new cell contact surfaces are formed. “We know that cells have the ability to sense the forces around them and tune their signalling pathways in response to these forces," Sugimura says. "Our data illustrate that AIP1 and cofilin are sensing tissue tension via structural changes of actin filament and that these actin binding proteins promote directional cell rearrangement by reinforcing the structural stability of remodelling cell contact surfaces.” The conversion of tissue tension into local actin turnover using AIP1 could be a strategy found in cell arrangement elsewhere in nature, as the protein is found in species from yeast to humans. The authors propose the mechanisms identified should be investigated in other tissue, as well as in other developmental contexts, such as cell proliferation and death. For more information about this research, contact Kaoru Sugimura [email protected] About Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS) At iCeMS, our mission is to explore the secrets of life by creating compounds to control cells, and further down the road to create life-inspired super materials that confront the myriad problems that afflict modern society. In only a decade, collaborative research at iCeMS has resulted in significant cutting-edge scientific discoveries, and the creation of over 1500 unique materials. We will keep turning our inspirations into purposeful, transformative innovations for the practical benefit of society. For more information about iCeMS, contact Mari Toyama [email protected]