Professor Wong Man-shing (right), Dr Li Hung-wing (second from left) and their team members.
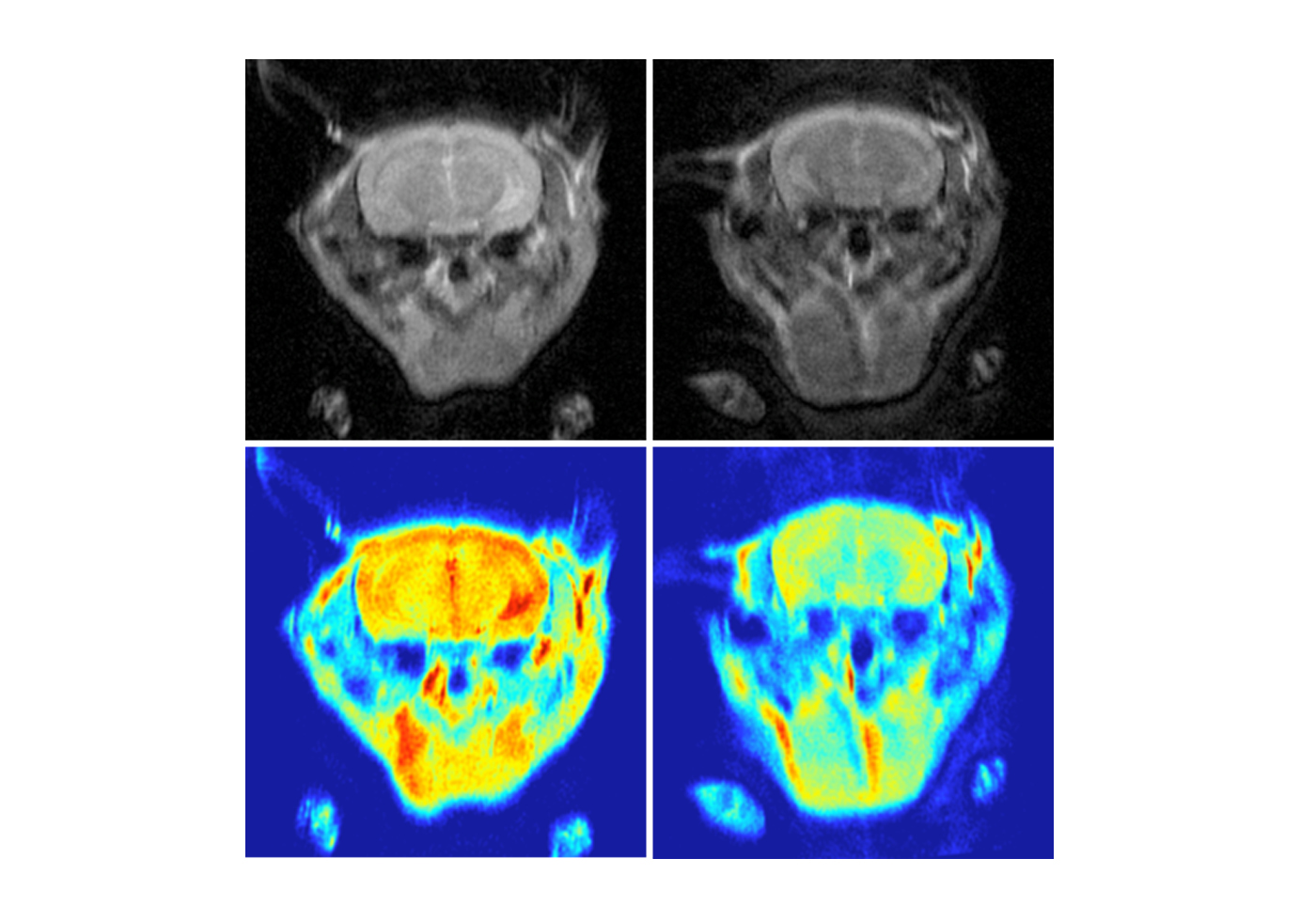
Researchers from Hong Kong Baptist University (HKBU) have discovered a new nanomaterial which could enable the early detection and diagnosis of Alzheimer’s disease. Non-toxic and sensitive to detection, the new material can easily pass through the blood-brain barrier*, enabling clear imaging – and detection – of the protein plaques which cause Alzheimer’s disease. The nanomaterial also shows a potent neuroprotective effect against the toxic protein. The team is jointly led by Professor Ricky Wong Man-shing and Associate Professor Dr Li Hung-wing from the Department of Chemistry at HKBU. The new nanomaterial could also be used to monitor the progression of the disease and the effectiveness of potential drugs. The study is published in the journal Small, and the discovery has already been granted a US patent. The plaques in the brain, which comprised of a protein called amyloid-beta, are one of the hallmarks of the Alzheimer’s disease. The early detection of these plaques could help speed up the diagnosis of Alzheimer’s and enable people to receive treatment earlier. Fabricated using a combination of superparamagnetic iron oxide nanoparticles and cyanine compounds, the team found that the nanomaterial could easily pass through the blood-brain barrier to specifically target these amyloid-beta plaques. Once bound to amyloid-beta, the nanomaterial fluoresces and exhibits magnetic resonance properties, enabling it to be easily detected by magnetic resonance imaging (MRI) and near-infrared imaging (NIRI) machines, which offer superior resolution and do not require an invasive radioactive trace. Professor Wong said: “Alzheimer’s disease is the most common cause of dementia, and it can lead to the loss of intellectual and social skills. The successful diagnosis of the disease at an early stage may help delay the disease’s progression, but current clinical methods of brain imaging using Positron Emission Tomography (PET) scans are expensive, require invasive radiative tracers and have poor visibility. “The fact that the new nanomaterial we have discovered is non-radioactive, non-toxic and able to penetrate the blood-brain barrier shows its promise for use in near-infrared imaging (NIRI) and MRI scanning of the brain. As a result, its application as a contrast agent for imaging is highly important and could lead to earlier detection – and improved monitoring – of Alzhimer’s disease.” The research work was financially supported by Collaborative Research Fund of the Research Grants Council. For more details, please watch the video (link below). *blood-brain barrier: the highly selective semipermeable membrane barrier that separates the circulating blood from the brain and extracellular fluid in the central nervous system which protects the brain from damage. Media enquiries: Professor Wong Man-shing of the Department of Chemistry (3411 7069, [email protected]) or Tina Ng of the Communication and Public Relations Office (3411 5262, [email protected]).