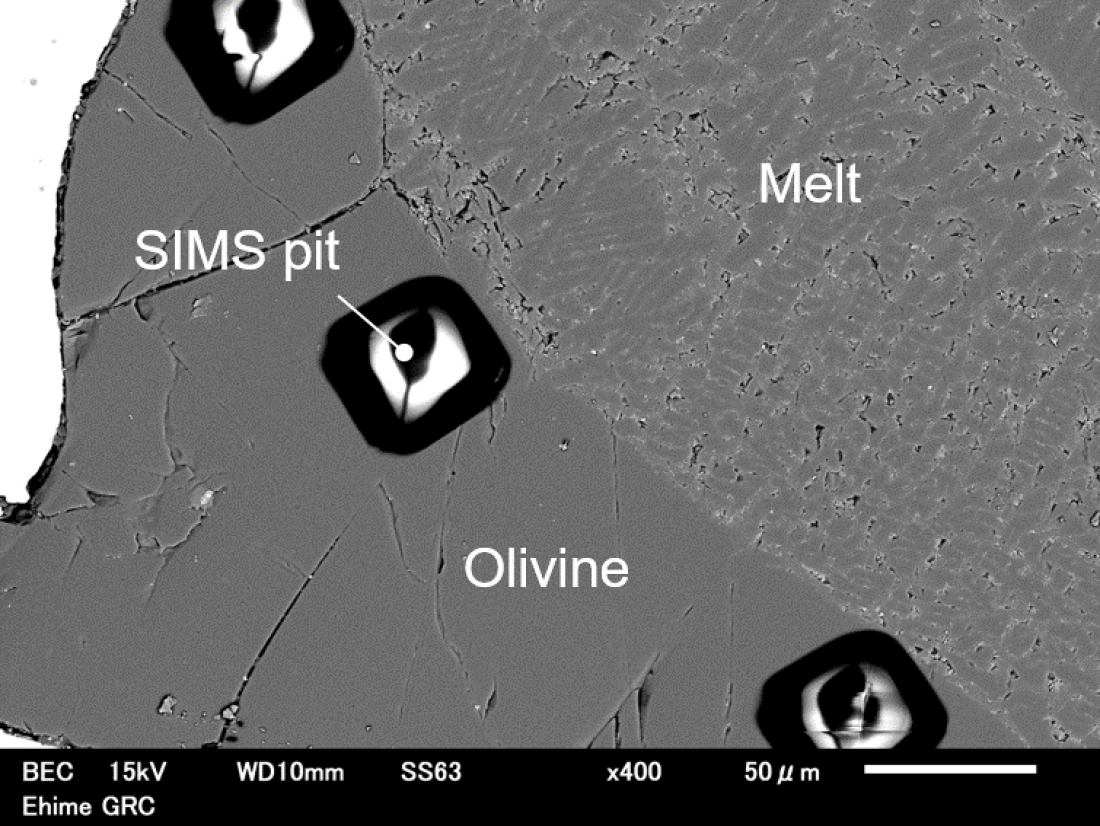
Melt is composed of a needle-like quench crystal and glass. Dark square regions are analytical pits of secondary ion mass spectrometry (SIMS).
Primitive chondrites, un-molten stony meteorites, are believed to be the building blocks of the Earth. Because terrestrial planets have experienced chemical differentiation in the core, mantle, and hydrosphere, the elemental abundance pattern of some elements at the planetary surface is not chondritic. In other words, the non-chondritic abundance pattern of elements on the planetary surface is a key to understanding the chemical differentiation processes of terrestrial planets.
It has been reported that the ratio of fluorine to chlorine in the silicate Earth (mantle + hydrosphere) is super-chondritic. This indicates an enrichment of fluorine in the silicate Earth compared to chlorine during and/or after the formation of the Earth. However, the processes which produced the super-chondritic F/Cl ratio of the Earth are poorly understood. In order to investigate the origin of the non-chondritic F/Cl ratio of the Earth, the research group of Ehime University and the University of Tokyo experimentally simulated fluorine and chlorine fractionation during magma ocean crystallization using a high-pressure apparatus (Kuwahara et al., 2019). The researchers found that fluorine was moderately compatible with bridgmanite, the most dominant mineral in the Earth’s mantle, but chlorine was highly incompatible with mantle minerals, including bridgmanite. This indicates that the crystallized mantle, resulting from a magma ocean, would have been enriched in fluorine, and chlorine may have become concentrated in the planetary surface.
After magma ocean crystallization, how was the super-chondritic F/Cl ratio in the silicate Earth established? Kuwahara et al. (2019) have proposed the escape of the hydrosphere during the formation of the Earth. In this scenario, chlorine is selectively lost into space while fluorine is retained in the silicate Earth, elevating the F/Cl ratio. Interestingly, previous studies have also proposed the same scenario to explain the Ar/Xe ratio of the silicate Earth (Shcheka and Keppler, 2012). These results suggest that the earliest atmosphere and, perhaps, ocean of the Earth may not have survived. If this is the case, the current Earth’s atmosphere and ocean might both be the second, having their origins in mantle degassing and/or impact delivery of volatiles after the formation of the Earth.