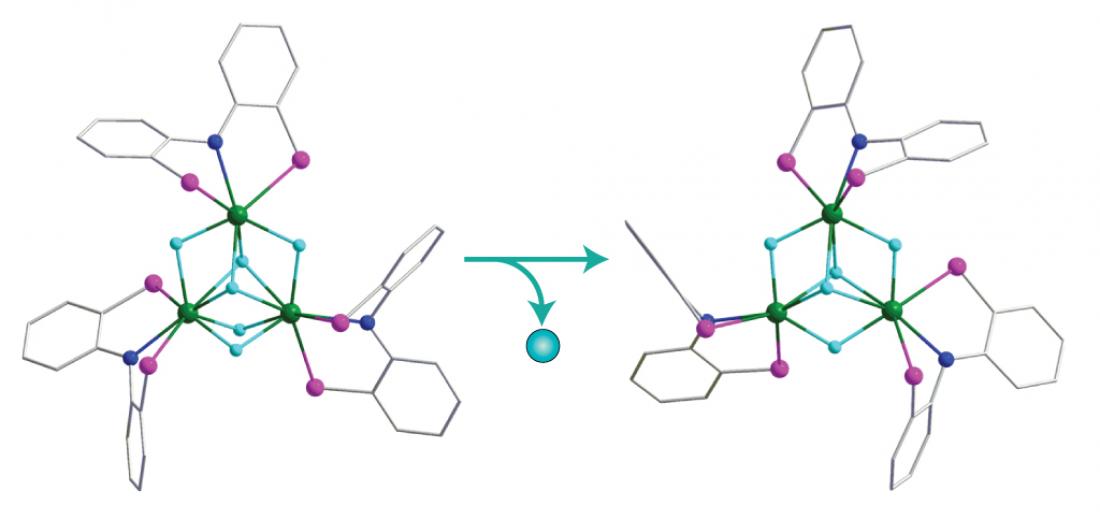
Figure 1: Bulky molecules known as PNP ligands help stabilize a novel rare earth polyhydride (left) as well as its cationic species (right) formed by removal of one hydrogen atom. Balls in green: yttrium; blue: nitrogen, pink: phosphorus, light-blue: hydrogen.
Rare-earth metals are a series of elements that represent one of the final frontiers of chemical exploration. The vigorous reactivity of these substances, however, has made it difficult for researchers to transform them into stable materials with well-defined structures. But when they succeed, the payoff can be enormous—rare-earth compounds have important applications in areas ranging from catalysis to clean energy.
Now, Zhaomin Hou and colleagues from the RIKEN Advanced Science Institute in Wako have discovered a new way to isolate rare-earth metals as hydrogen-infused crystals by using wedge-shaped bis(phosphinophenyl)amido (PNP) ligands to ‘pinch’ them in place[1]. These ligands squeeze rare-earth yttrium atoms together tighter than any previous material, and can even stabilize highly volatile charged complexes.
Metallic compounds that incorporate multiple hydrogen atoms, or polyhydrides, into their frameworks are useful to chemists because they provide some of the purest understandings of bonding and reactivity available. Previously, Hou’s team isolated an yttrium polyhydride containing a hydrogen ligand that simultaneously bonds to four metals[2]. This compound sparked remarkable chemical curiosity because of its structural novelty.
According to Hou, the trick to producing rare-earth polyhydrides is to surround them with large, cumbersome molecules that easily pack together to form crystals. The distinct structure of PNP ligands—two phosphorus atoms, linked together by a rigid aromatic–amino core that can bind to metals with a pincer-like grip—made this ligand a promising candidate for the researchers’ investigation.
By first substituting extra methyl units onto the aromatic backbone of PNP to increase its bulkiness, and then mixing the ligand with an yttrium alkyl precursor and hydrogen gas, the team synthesized pale yellow crystals of a new yttrium polyhydride complex. X-ray structural analysis revealed that three yttrium atoms, held in place by PNP ‘pincers’, were interlinked by a set of double- and triple-bridged hydrogen ligands (Fig. 1). This intricate network of bonds produced the shortest yttrium–yttrium distance ever recorded—an extraordinary packing density that may be critical for future hydrogen-storage applications.
The researchers found that an ammonium proton could remove a hydride from the complex without disrupting crystallization, yielding the first-ever cationic tri- and di-yttrium polyhydrides. The charged nature of these materials should impart potent chemical activity, attributes which Hou and his team are currently investigating. “Our results clearly demonstrate the vital importance of ligand-tuning in the isolation and characterization of rare earth polyhydrides, and should encourage further explorations in this burgeoning area,” he says.
The corresponding author for this highlight is based at the Organometallic Chemistry Laboratory, RIKEN Advanced Science Institute