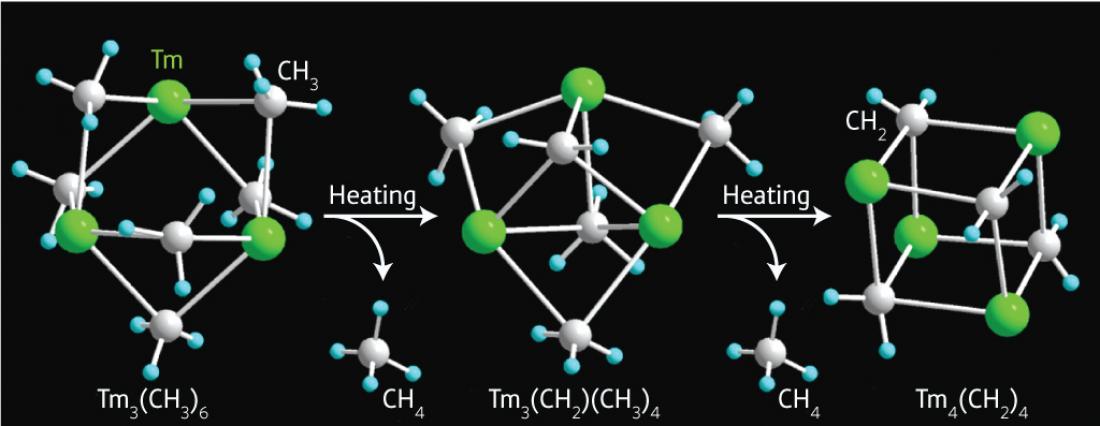
Figure 1: Eliminating methane (CH4) molecules from polyhedral thulium–methyl crystals (Tm3(CH3)6) (left and center) produces a new cube-shaped rare earth–carbene complex (right) with intriguing chemical behavior.
In synthetic chemistry, ‘carbene’ species—compounds bearing a carbon atom with two unpaired electrons—have a ferocious reputation. Left uncontrolled, they will react with almost any molecule they meet. But by harnessing this vigor with transition metals, chemists can turn carbenes into powerful chemical transformation reagents. Now, Zhaomin Hou and colleagues from the RIKEN Advanced Science Institute in Wako report a new class of compounds that contain multiple carbene units in one extraordinary structure: a cube-shaped molecule stabilized by ligand-protected rare-earth metals[1].
Rare-earth metals hold more electrons within their atomic radii than most other elements, making them essential in high-tech devices such as superconductors and hybrid vehicle batteries. Combining these metals with carbenes could lead to breakthrough procedures in synthetic chemistry. However, rare-earth metal–carbene complexes are usually unstable because the bonds they form are lopsided electronically, and therefore extremely reactive.
To overcome this problem, Hou and colleagues turned to a bulky ligand, based on a five-membered aromatic ring called cyclopentadiene (Cp´), which can trap rare-earth metal–carbene complexes into ordered solids. By mixing Cp´-protected lutetium (Lu) and thulium (Tm) rare-earth metal precursors with a carbon-donating aluminum reagent, they isolated a unique set of hybrid polyhedral crystals. X-ray analysis showed that these materials had a core of three rare-earth metals interconnected by six bridging methyl (CH3) groups.
An unexpected twist occurred when the researchers tested the thermal stability of the Lu– and Tm–methyl complexes. Heating to 90 °C caused the methyl groups to lose one of their hydrogen atoms, transforming them into carbenes. Then, after the elimination of a methane molecule, the crystal structure rearranged into a perfectly shaped cube featuring four Cp´-protected rare-earth metals and four carbene units (Fig. 1).
The team’s experiments revealed that the cubes spontaneously turned benzene–carbonyl molecules into alkenes by swapping their carbene groups for oxygen atoms, yielding a new oxygenated cube in the process. The researchers are now examining the reactivity of the cubes toward other molecules and plan to fine-tune the structure and reactivity of carbene compounds by investigating differently sized rare-earth metals together with different supporting ligands.
“This work demonstrates for the first time that methane can be eliminated rather easily from rare earth complexes containing methyl groups, affording structurally stable but highly reactive multi-carbene species,” says Hou. “Further studies along this line should open up a completely new frontier in rare-earth carbene chemistry.”
The corresponding author for this highlight is based at the Advanced Catalyst Research Team, RIKEN Advanced Science Institute