Figure 1: Reversible hydrogen addition and release of heterometallic polyhydride clusters (top). The structure changes of the hydride clusters resemble the up-down movement of the pantagraph of a train (bottom).
The most abundant element in the universe, hydrogen holds great promise as a source of clean, renewable energy, producing nothing but water as a byproduct and thus avoiding the environmental dangers associated with existing mainstream energy sources. Broad adoption of hydrogen, however, has stalled because in its natural gaseous state, the element simply takes up too much space to store and transport efficiently.
One way to solve this problem is to use metal hydrides, metallic compounds that incorporate hydrogen atoms, as a storage medium for hydrogen. In this technique, the metal hydrides bind to hydrogen to produce a solid one thousand times or more smaller than the original hydrogen gas. The hydrogen can then later be released from the solid by heating it to a given temperature.
The new heterometallic hydride clusters synthesized by the RIKEN researchers use rare-earth and d-transition metals as building blocks and exploit the advantages of both. Rare earth metal hydrides remove one major obstacle by enabling analysis using X-ray diffraction, a technique which is infeasible for most other metal hydrides – offering unique insights into underlying reaction processes involved. Rare earth metal hydrides on their own, however, do not undergo reversible hydrogen addition and release, the cornerstone of hydrogen storage. This becomes possible through the addition of a d-transition metal, in this case tungsten (W) or molybdenum (Mo).
While rare-earth / d-transition metal-type metallic hydride complexes have been studied in the past, the current research is the first to explore complexes with multiple rare earth atoms of the form Ln4MHn and with well-defined structures (Ln = a rare-earth metal such as yttrium, M = a d-transition metal, either tungsten or molybdenum, and H = hydrogen). In a paper in Nature Chemistry, the researchers show that these complexes exhibit unique reactivity properties, pointing the way to new hydrogen storage techniques and promising environmentally-friendly solutions to today’s pressing energy needs.
For more information, please contact:
Zhaomin Hou
Organometallic Chemistry Laboratory
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-9393 / Fax: +81-(0)48-462-4665
Takanori Shima
Organometallic Chemistry Laboratory
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-9392 / Fax: +81-(0)48-462-4665
Global Relations Office
RIKEN
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: [email protected]
Reach us on Twitter: @rikenresearch
Reference
Takanori Shima, Yi Luo, Timothy Stewart, Robert Bau, Garry J. McIntyre, Sax A. Mason and Zhaomin Hou. "Molecular heterometallic hydride clusters composed of rare-earth and d-transition metals." Nature Chemistry, 2011, DOI: 10.1038/NCHEM.1147
########################################################################################################
About RIKEN
RIKEN is Japan’s flagship research institute devoted to basic and applied research. Over 2500 papers by RIKEN researchers are published every year in reputable scientific and technical journals, covering topics ranging across a broad spectrum of disciplines including physics, chemistry, biology, medical science and engineering. RIKEN’s advanced research environment and strong emphasis on interdisciplinary collaboration has earned itself an unparalleled reputation for scientific excellence in Japan and around the world.
About the Advanced Science Institute
The RIKEN Advanced Science Institute (ASI) is an interdisciplinary research institute devoted to fostering creative, curiosity-driven basic research and sowing the seeds for innovative new projects. With more than 700 full-time researchers, the ASI acts as RIKEN’s research core, supporting inter-institutional and international collaboration and integrating diverse scientific fields including physics, chemistry, engineering, biology and medical science.
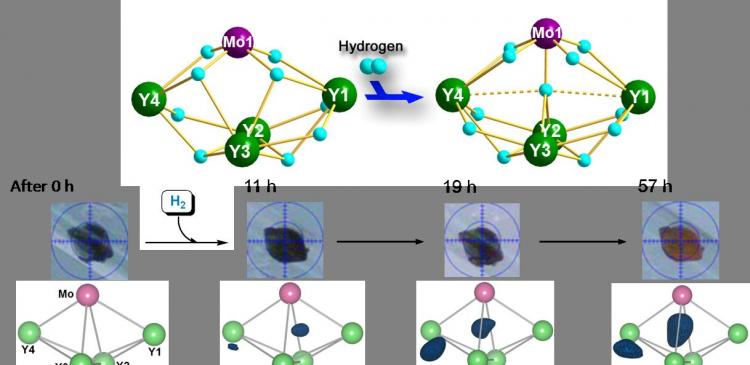
Figure 2: Addition of hydrogen to a metal hydride cluster with retention of the single crystal morphology. Top: A schematic representation of the hydrogen addition reaction. Middle: Photographs of the crystal at different reaction times. The crystal color changed from black to red as the reaction proceeded. Bottom: Differential electron density maps showing the appearance and electron density increase of the two new hydride ligands (blue) as the hydrogenation reaction proceeded.