Background to the research
Marfan’s syndrome (MFS) is a severe, systemic disorder of connective tissue formation and can lead to aortic aneurysms, ocular lens dislocation, emphysema, bone overgrowth and severe periodontal disease. MFS has an estimated prevalence of 1 in 5,000-10,000 individuals. Various mouse models of MFS have been established via gene targeting or missense mutations, with germline mutations in fibrillin-1 leading to progressive connective tissue destruction due to fibrillin-1 fragmentation in association with an insufficiency of fibrillin-1 microfibril formation. Hence, it is largely accepted that MFS is caused by insufficient fibrillin-1 microfibril formation in various connective tissues.
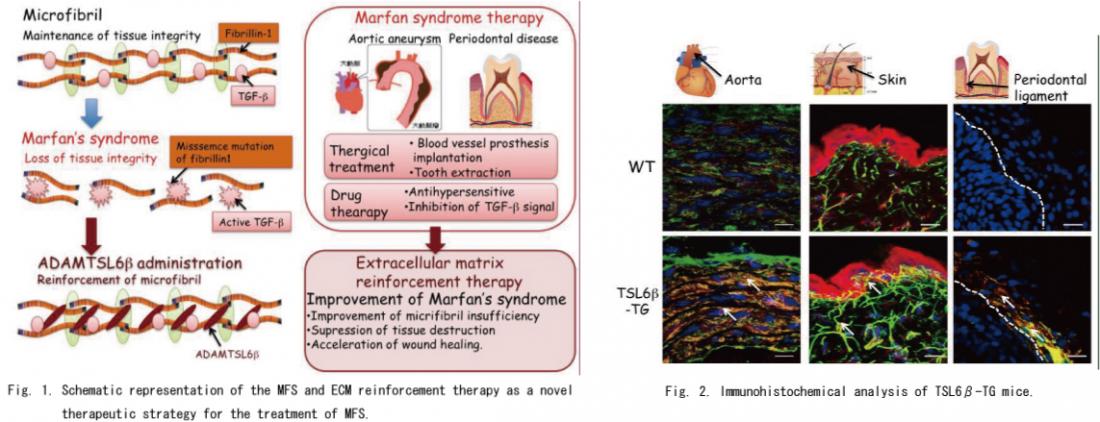
A variety of MFS therapies have been developed, including surgical therapy for aortic root aneurysms that are life-threatening, traditional medical therapies such as β-adrenergic receptor blockade for slow aortic growth and to decrease the risk of aortic dissection, and novel approaches based on new insights such as the pathogenesis of insufficient fibrillin-1 microfibril formation and deregulation of Transforming Growth Factor-type beta (TGF-β) activation. It has been demonstrated also that deregulation of (TGF-β) activation contributes to MFS pathogenesis and that matrix sequestration of TGF-β is critical for the regulated activation and signaling of the extracellular fibrillin-1 microfibrils of connective tissues. Importantly, systemic antagonism of TGF-β signaling through the administration of a TGF-β neutralizing antibody or losartan, an angiotensin II type 1 receptor blocker, has been shown to have a beneficial effect on alveolar septation and muscle hypoplasia. These observations provide a proof-of-principle for the use of TGF-β antagonism is a general therapeutic strategy for MFS and other disorders of the TGF-β signaling network. However, another potential therapeutic strategy which remains to be investigated is the reconstruction of the microfibril in connective tissues through the expression or administration of a microfibril-associated molecule that regulates or stabilizes fibrillin-1 microfibril formation. To investigate this concept, it will be necessary to identify molecular mechanisms of microfibril formation and an appropriate fibrillin-1 microfibril associated molecule (Fig .1).
Fig. 1. Schematic representation of the MFS and ECM reinforcement therapy as a novel therapeutic strategy for the treatment of MFS.
Left panel : Fibrillin-1 comprises insoluble extracellular matrix components in connective tissue microfibrils and provides limited elasticity to tissues through fibrillin-1 microfibril formation. Missense mutations of fibrillin-1 leading to progressive connective tissue destruction due to fibrillin-1 fragmentation in association with an insufficiency of fibrillin-1 microfibril formation. ADAMTSL6β is essential for fibrillin-1 microfibril formation and suggest a novel therapeutic approach to the treatment of MFS through the promotion of ADAMTSL6β-mediated fibrillin-1 microfibril assembly.
Right Panel : A variety of MFS therapies have been developed, including surgical therapy for aortic root aneurysms that are life-threatening, traditional medical therapies such as β-adrenergic receptor blockade for slow aortic growth and to decrease the risk of aortic dissection, and novel approaches based on new insights such as the deregulation of TGF-β activation. ECM reinforcement therapy which induces restoration of properly formed microfibrils by ADAMTSL6β is essential not only for improvement of the predominant symptoms of MFS, but also for the suppression of excessive TGF-β signaling induced by microfibril disassembly.
Outline of the research outcome
1. Identification of ADAMTSL6β which induces microfibril assembly.
The novel ADAMTSL family molecules ADAMTSL6α and 6β were recently identified by in silico screening for novel ECM proteins produced from a mouse full-length cDNA database (FANTOM). These proteins are localized in connective tissues, including the skin, aorta and perichondrocytes. Among the ADAMTSL6 family, ADAMTSL6β has been shown to associate with fibrillin-1 microfibrils through its direct interaction with the N-terminal region of fibrillin-1, and thereby promotes fibrillin-1 matrix assembly in vitro and in vivo. These findings suggest a potential clinical application of ADAMTSL6β as a novel MFS therapy by promoting fibrillin-1 microfibril assembly and regulating TGF-β activation.
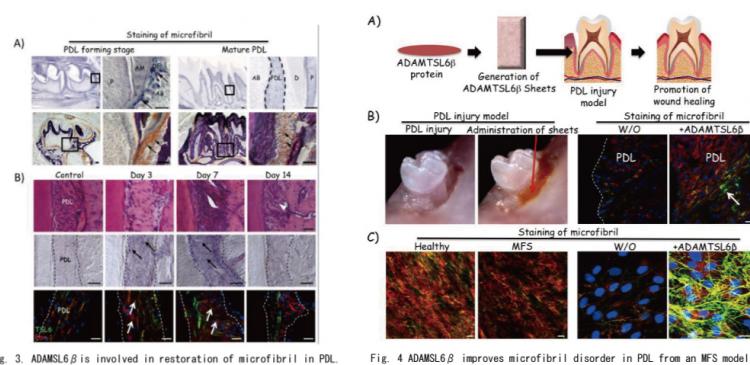
To investigate whether ADAMTSL6β plays a critical role in microfibril assembly in connective tissues, we generated ADAMTSL6β transgenic mice (TSL6βTGmice) in which the transgene is expressed in the whole body. Since ADAMTSL6β has been shown to be expressed in the aorta and skin, we investigated microfibril assembly of these tissues in the TSL6βTGmice. Immunohistochemical analysis revealed that ADAMTSL6β positive microfibril assembly was barely detectable in WT mice but strongly induced in the aorta of TSL6βTGmice (Fig. 2). Histological analysis revealed that microfibrils are clearly increased in the aorta and that microfibril assembly is also induced in the skin and PDL of TSL6βTG. mice. This confirmed that ADAMTSL6β induces fibrillin-1 microfibril assembly in connective tissue such as the aorta, skin and PDL.
Fig. 2. Immunohistochemical analysis of TSL6β-TG mice. Cryosections were prepared from the aortas (left), skin (middle) or PDL (right) of wild type (upper panel) or TSL6βTG (lower panel) littermates and subjected to double immunostaining with antibodies against ADAMTSL6β (red) and fibrillin-1 (green). ADAMTSL6β and fibrillin-1-positive microfibrils (green yellow) was markedly increased in the aorta and skin of TSL6β TG mice compared with WT mice. Bar=50 µm
2. ADAMTSL6. is involved in microfibril restration during PDL wound healing.
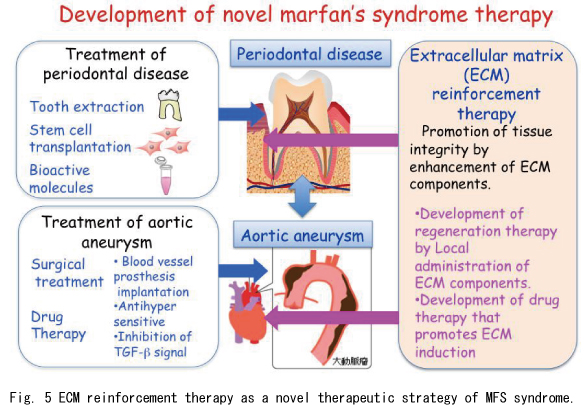
In our current study, we report that ADAMSL6β is essential for the development and regeneration of the connective tissue periodontal ligament (PDL), a tooth-supporting tissue located between the root and alveolar bone which is morphologically similar to the ligament tissue that is capable of withstanding mechanical force. To investigate whether ADAMSL6β contributes to connective tissue formation, we first examined its expression patterns during PDL formation stage after birth as a model of connective tissue formation. In situ hybridization analysis revealed that ADAMSL6β was strongly expressed in the PDL forming stage of the DF however ADAMSL6β expression was significantly downregulated in the adult PDL(Fig. 3A upper). Immunohistochemical analysis further revealed that ADAMSL6β is detectable in assembled microfibril-like structures during the PDL forming stage of the DF, and in organized microfibrils in the adult PDL (Fig. 3A lower). Because developmental processes involve similar mechanisms to wound healing, we next determined whether ADAMSL6β is involved in PDL microfibril assembly during wound healing using a tooth replantation model. Histochemical analysis revealed an injured PDL with an irregular architecture at 3 days after replantation, although gradual although gradual healing then occurred at between 7 and 14 days after replantation. During these processes, adamtsl6β mRNA expression were found to be clearly induced in the PDL at 3 to 7 days after replantation, but to decrease again by 14 days after replantation (Fig. 3B). Similar to these gene expression patterns, adamtsl6- and fibrillin-1-positive microfibrillar-like structures resembling those seen in the DF during the PDL forming stage were markedly increased in the damaged PDL at 3 to 7days after replantation.
Fig. 3. ADAMSL6β is involved in restoration of microfibril in PDL.
A) ADAMSL6β mRNA (arrows) by specific probes at the P1-late bell stage of dental follicle formation in the tooth germ is indicated by arrows (upper). Expression of ADAMSL6β microfibrils was detected in dental follicle during PDL forming stage is indicated by arrows (lower).
B) Histological analysis of injured PDL 3 days, 7 days and 14 days after replantation of the tooth were analyzed by histological analysis (upper). Histological analysis indicated that expression of ADAMSL6β mRNA were markedly increased 3 days and 7 days after injury (middle). Immunohistochemical analysis using anti-ADAMSL6β (TSL6 : green) and anti-fibrillin-1 (Fbn-1: red) antibodies indicated that expression of ADAMSL6β- and fibrillin-1-positive microfibrils was markedly increased 3 days and 7 days after injury (Lower). AB:alveolar bone, AM:ameloblast, PDL:periodontal ligament, D:dentin, P:pulp
3. The local administration of ADAMTSL6β improves wound healing ability in a MFS model.
We next investigated whether ADAMTSL6β might be developed as a novel therapeutic for MFS microfibril disorder. Collagen gel containing recombinant ADAMSL6β was locally administrated into an experimentally damaged PDL in MFS mice model (Fig. 4A, B left). Histochemical analysis showed that a reorganization of microfibril assembly and wound healing could be observed after 17 days of incubation (Fig. 4B right). In contrast, the administration of control collagen gel failed to induce PDL healing and microfibril formation (Fig. 4B right ).
We next investigated whether ADAMTSL6β alleviates fibrillin-1 microfibril disorder in periodontal ligament cells obtained from MFS patient (MHPDL), which shows a reduction in fibrillin-1 microfibril assembly (Fig. 4C left). We next investigated whether recombinant ADAMSL6β improves the symptoms of MHPDL microfibril disorder. We found that recombinant ADAMSL6β induces microfibril assembly in MHPDL cells during three days incubation in culture (Fig. 4C). These results illustrate that reorganization of microfibrils by recombinant ADAMSL6β improves structurally damaged microfibril in MFS.
Fig. 4 ADAMSL6β improves microfibril disorder in PDL from an MFS model.
A) Schematic representation of the local administration of recombinant ADAMSL6β into a PDL injury model
B) After injury of PDL by dislocation, collagen gel-containing recombinant ADAMSL6β was then injected into the injured PDL (left). Immunohistochemical analysis showed an improvement in fibrillin-1 microfibril assembly (arrowheads) induced by the injection of recombinant ADAMSL6β. WO:Without treatment of ADAMSL6β.
C) Histological analysis of PDL cells obtained from MFS treated with recombinant ADAMTSL6β. PDL cells obtained from MFS patients showed microfibril insufficiency compared with PDL cells obtained from healthy patient (left). Administration of ADAMSL6βmarked improvement of microfibril assembly in PDL cells obtained from MFS patients (right).
In conclusion, we provide evidence for the contributions of ADAMTSL6β-mediated fibrillin-1 microfibril assembly to PDL development, regeneration, and alleviation of MFS manifestations. Wethereby introduce the concept that an ECM reinforcement therapy such as ADAMTSL6β administration which induces microfibril assembly, should be considered in the development of future mechanism-based therapeutics for the improvement of connective tissue disorders such as MFS. Our data suggest that the reinforcement of fibrillin-1 assembly by ADAMTSL6β accelerates the sequestration of newly synthesized TGF-β. It will also be necessary to develop methodologies for the systemic administration of ADAMTSL6β to induces microfibril assembly in connective tissue for the treatment of life-threatening conditions such as aortic aneurysm. Since elastolysis occurs continuously in aortic aneurysms in MFS, chronic administration of ADAMTSL6βmay be required for the stabilization of microfibrils to prevent progressive tissue destruction. This approach will facilitate drug discovery for treating MFS and related connective tissue disorders.
Fig. 5 ECM reinforcement therapy as a novel therapeutic strategy of MFS syndrome. ECM reinforcement therapy such as ADAMTSL6β administration which induces microfibril assembly, should be considered in the development of future mechanism-based therapeutics for the improvement of connective tissue disorders such as MFS.