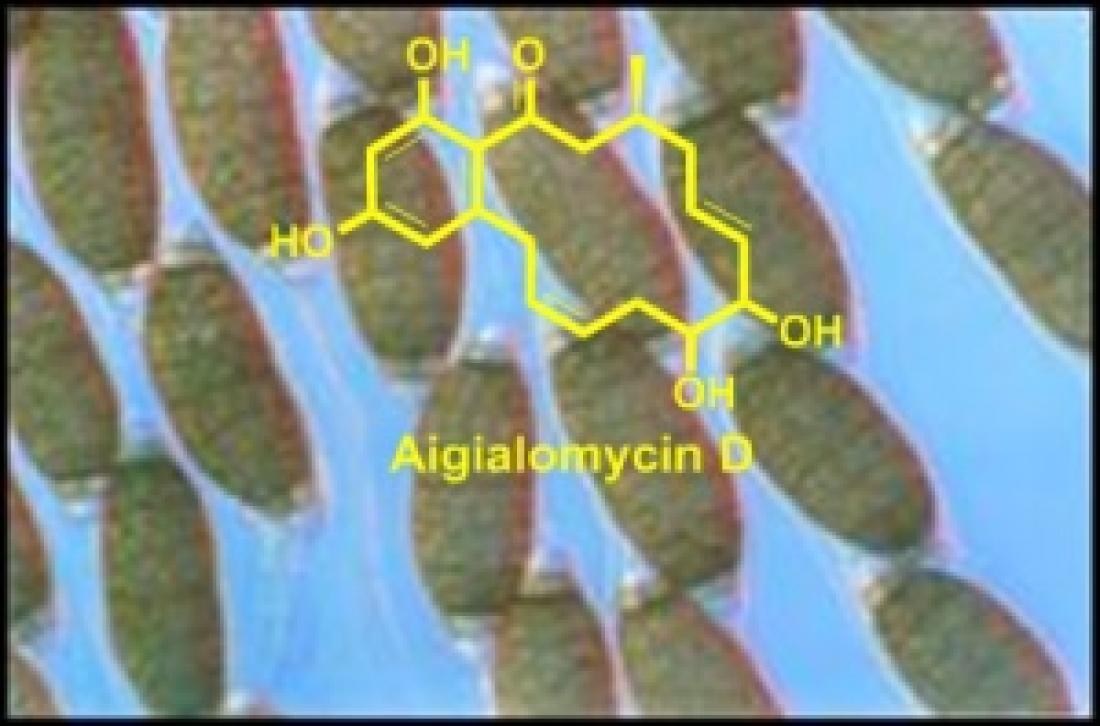
Aigialomycin D, a promising kinase inhibitor, is derived from the fungus Aigialus parvus
Nature has provided scientists with an abundant pharmacopeia for fighting diseases and cancer. For example, recent studies have identified several resorcylic acid lactones — compounds derived from mangrove fungi — as potent inhibitors of enzymes that drive tumor growth.
Anqi Chen and Christina Chai at the A*STAR Institute of Chemical and Engineering Sciences have been particularly interested in the properties of one such resorcylic acid lactone, aigialomycin D (see image).
“Aigialomycin D has shown good cytotoxicity against some cancer cell lines, but little is known about the structure-activity relationship of this resorcylic acid lactone,” says Chen. “We decided to capitalize on this by synthesizing a series of compounds related to aigialomycin D so that we could identify potential leads for the development of new cancer drugs.”
Chen, Chai and their team have examined the biochemical properties of aigialomycin D and dozens of its derivatives1. Aigialomycin D differs from other resorcylic acid lactones in the absence of a chemical group that is thought to play an important role in the inhibition of cancer, but the researchers nevertheless confirmed that aigialomycin D and several derivatives modestly inhibited cyclin-dependent kinase 2 (CDK2), a key enzyme in driving tumor growth. More importantly, subsequent screening against a panel of nearly 100 different kinases revealed at least five that were notably inhibited by aigialomycin D.
Among the most interesting of these was an enzyme called mitogen-activated protein kinase-activating kinase 2 (MNK2), which was strongly inhibited by aigialomycin D. MNK2 and its sister enzyme MNK1 selectively activate proteins that promote cancer proliferation. Although neither protein has been solidly confirmed as a causative factor in cancer, excessive MNK activity has been implicated to be a tumor-specific phenomenon. Several other enzymes identified in the screen also represent promising targets, such as FMS-like tyrosine kinase 3 (FLT3), which is a causative factor in several blood cancers. “Over-expression of these enzymes has been shown in several cancers,” says Chen. “The discovery of aigialomycin D as a potent inhibitor for these enzymes could serve as a starting point for the discovery of new anticancer drugs.”
Chen, Chai and their team would like the molecule to have better specificity and efficacy, so they are going back to the drawing board. “We will synthesize more analogues of resorcylic acid lactones to identify better leads,” says Chen. “The medium-term plan would then be to optimize promising leads to achieve better drug-like properties that can be brought forward for further development.”
----------------------------
The A*STAR-affiliated researchers contributing to this research are from the Institute of Chemical and Engineering Sciences
References
Xu, J. et al. Exploring aigialomycin D and its analogues as protein kinase inhibitors for cancer targets. ACS Medicinal Chemistry Letters 2, 662–666 (2011). | article