Image 1: The IBN research team that developed this device comprises Executive Director Professor Jackie Y. Ying, Postdoctoral Fellow Dr Yi Zhang and Research Scientist Dr Jianhao Bai (from left to right).
Singapore, 29 January 2015 – Finding out whether you have been infected with dengue may soon be as easy as spitting into a rapid test kit. The Institute of Bioengineering and Nanotechnology (IBN) of A*STAR has developed a paper-based disposable device that will allow dengue-specific antibodies to be detected easily from saliva within 20 minutes. This device is currently undergoing further development for commercialization.
IBN Executive Director Professor Jackie Y. Ying shared, “Our rapid diagnostic kit can detect a key dengue antibody from saliva that is present in early-stage secondary infection. The ability to differentiate between primary and secondary dengue infections makes it a valuable early diagnosis tool that would help to ensure timely treatment and proper care of patients.”
Patients with secondary infection, who have previously been infected with other serotypes of dengue virus, stand a higher risk of developing dengue hemorrhagic fever or dengue shock syndrome.
According to Singapore’s National Environment Agency, dengue fever and its more severe form, dengue hemorrhagic fever, are the most common mosquito-borne viral diseases in the world. This disease poses a serious health threat, and is a leading cause of illness and death in tropical and subtropical climates. There are four known serotypes of the dengue virus, but no vaccine or medicine has been developed to treat the illness. The incubation period before symptoms develop generally ranges from 4 to 10 days after infection. Therefore, early diagnosis would enable the patient to receive prompt medical attention and avoid further complications.
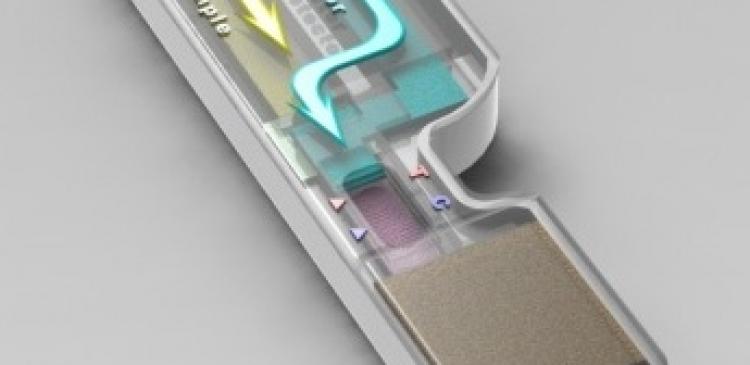
Currently, dengue infection is diagnosed in the laboratory by testing the patient’s blood sample for the presence of dengue antigens or antibodies. IBN’s device, on the other hand, is capable of detecting IgG, a dengue-specific antibody found at the onset of secondary infections, directly from saliva in one step.
Unlike blood samples, saliva can be collected easily and painlessly for rapid point-of-care diagnostics. However, unlike other body fluids, it cannot be applied directly to commercially available test kits as it would cause the sensor nanoparticles to stick haphazardly to the test strip. In addition, conventional paper-based tests are not designed to handle the larger sample volume of saliva required.
As described in the journal Lab on a Chip, the IBN researchers used an innovative stacking flow design to overcome key challenges faced by existing lateral flow designs, such as those used in pregnancy test kits.
In IBN’s device, different flow paths are created for samples and reagents through a multiple stacked system. This allows the saliva sample to flow separately through a fiber glass matrix, which removes the substances that would interfere with the nanoparticle-based sensing system before it mixes with the sensor nanoparticles. IBN’s device configuration also helps to regulate the flow in the test strip, generating uniform test lines for more accurate results.
By simplifying the diagnostic procedure, the researchers hope to make the device as easy to use as over-the-counter pregnancy or fertility test kits. IBN’s oral test kit may be adapted to detect other infectious diseases. The IBN researchers are also investigating the use of other common fluid samples, such as blood, urine and serum for rapid, high-sensitivity test kits.
The Institute is currently collaborating with ARKRAY Inc., a pioneer in the field of automated analysis systems, to commercialize its paper-based diagnostic technology. In 2013, ARKRAY opened its first Asian research center outside Japan in IBN with an investment of S$9.1 million over five years. The research center is focused on developing novel detection kits for infectious diseases based on IBN’s innovative diagnostic platforms.
Mr. Atsushi Murakami, General Manager of the R&D Division of ARKRAY Inc., said: “We have developed an excellent working relationship with IBN over the past two years, and our research activities have progressed rapidly. Together, we will continue to focus on the successful commercialization of new technologies for the diagnosis of tropical infectious diseases.”
IBN has been focused on research in medical technologies since 2003, and has created unique devices and assays for the rapid and accurate diagnosis of diseases. The institute has an active portfolio of 500 patents/patent applications, of which 86 have been licensed. It has spun off seven companies in the medical technology sector and its innovations have attracted major interest from the industry.
Reference:
1. Y. Zhang, J. Bai and J. Y. Ying, “A Stacking Flow Immunoassay for the Detection of Dengue-Specific Immunoglobins in Salivary Fluid,” Lab on a Chip, (2015) DOI: 10.1039/C4LC01127A.
Media Contacts:
Nidyah Sani
Phone: +65 6824 7005
Email: [email protected]
Elena Tan
Phone: +65 6824 7032
Email: [email protected]
About the Institute of Bioengineering and Nanotechnology
Established in 2003, the Institute of Bioengineering and Nanotechnology (IBN) is the world’s first bioengineering and nanotechnology research institute. IBN’s mission is to conduct multidisciplinary research across science, engineering, and medicine for breakthroughs to improve healthcare and quality of life.
IBN’s research activities are focused in the following areas:
Nanomedicine, where functionalized polymers, hydrogels and biologics are developed as therapeutics and carriers for the controlled release and targeted delivery of therapeutics to diseased cells and organs.
Cell and Tissue Engineering, where biomimicking materials, stem cell technology, microfluidic systems and bioimaging tools are combined to develop novel approaches to regenerative medicine and artificial organs.
Biodevices and Diagnostics, which involve nanotechnology and microfabricated platforms for high-throughput biomarker and drug screening, automated biologics synthesis, and rapid disease diagnosis.
Green Chemistry and Energy, which encompass the green synthesis of chemicals and pharmaceuticals, catalytic conversion of biomass, utilization of carbon dioxide, and new nanocomposite materials for energy applications.
Scientific Impact
More than 1,000 papers published in leading scientific journals
Over 1,100 seminars and presentations at international conferences, including over 700 invited, keynote and plenary lectures
Organized premier scientific meetings such as the International Conference on Bioengineering and Nanotechnology, Nano Today Conference, and the IBN International Symposium
Technological and Commercialization Impact
500 active patents and patent applications
86 licensed patents and patent applications
7 spin-off companies
140 active research collaborations with industrial, clinical and academic partners
Nurturing Future Research Talents
Trained 106 PhD students
More than 77,700 students and teachers from 290 local and overseas schools/universities have participated in IBN’s Youth Research Program
Over 2,000 students and teachers have completed research attachments at IBN
For more information about IBN, please visit www.ibn.a-star.edu.sg.
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that fosters world-class scientific research and talent to drive economic growth and transform Singapore into a vibrant knowledge-based and innovation driven economy.
In line with its mission-oriented mandate, A*STAR spearheads research and development in fields that are essential to growing Singapore’s manufacturing sector and catalyzing new growth industries. A*STAR supports these economic clusters by providing intellectual, human and industrial capital to its partners in industry.
A*STAR oversees 18 biomedical sciences and physical sciences and engineering research entities, located in Biopolis and Fusionopolis, as well as their vicinity. These two R&D hubs house a bustling and diverse community of local and international research scientists and engineers from A*STAR’s research entities as well as a growing number of corporate laboratories.
For more information on A*STAR, please visit www.a-star.edu.sg