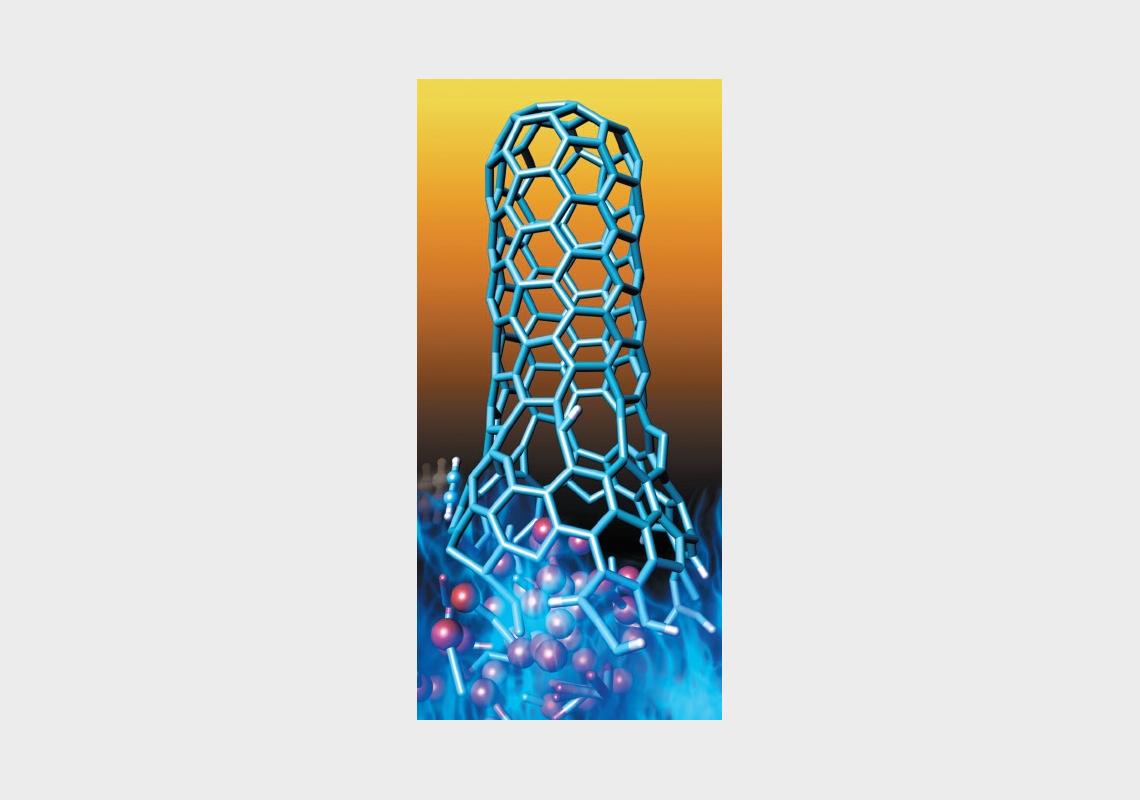
The synthesis of carbon nanotubes (CNTs), with a view to their industrial-scale production, is attracting heavy scientific interest. Their unique chemical properties promise a wide variety of groundbreaking uses in acoustic, biomedical, electronic, environmental, optical and structural technologies.
Led by Professor Stephan Irle of the Institute of Transformative Bio-Molecules at Nagoya University, a team of researchers in Japan, the United States and China conducted computer simulations that show similar molecular mechanisms at work in the growth of carbon nanotubes and the combustion of hydrocarbons to form soot. This discovery challenges a previously accepted view that metal carbides are needed to create nanotubes, through a process called chemical vapour deposition.
The team’s own models suggest that alternative chemical processes such as hydrogen- bstraction/acetylene addition – a mechanism often observed in combustion processes – could also be used to grow carbon nanotubes. “This finding is very intriguing in the sense that these processes were long considered to proceed by completely different mechanisms,” says Professor Irle.
In 2014, the team reported the first growth simulations of single-walled carbon nanotube synthesis, using acetylene as a feedstock. Their simulation model used acetylene due to the relatively low temperatures needed to catalyse chemical vapour deposition, and because even small amounts speed up reactions significantly. According to the researchers, their modelling suggests that acetylene’s potential role needs further study, as does the currently accepted model for carbon nanotube production.
Since publishing their results, the researchers have started modelling the synthesis of graphene – a one atomthick layer of pure carbon – using nickel and copper with a methane catalyst. They hope to publicly release the new simulation model – based on a direct version of a kinetic Monte Carlo simulation where reaction channels are predicted automatically on the fly as the growth process proceeds – in 2015.
DID YOU KNOW?
Carbon nanotubes are nanocylinders consisting of one atom-thick sheets of carbon (or graphene). They are currently used as additives to strengthen various structural materials, and may also be used for energy storage as well as in the next generation of nanoelectronics and biomedical devices. CNTs are often synthesised via chemical vapour deposition, in which hydrocarbon vapour is deposited on metal catalysts under a flow of non-reactive gas at high temperatures. However, quality control is a challenge since this method usually results in the production of CNTs with variable diameters and different sidewall structures.
For further information contact:
Professor Stephan Irle
Institute of Transformative Bio-Molecules
World Premier International Research Center Initiative
Nagoya University, Japan
E-mail: [email protected]
Institute of Transformative Bio-Molecules (ITbM), Nagoya University
*This article also appears in Asia Research News 2015 (P.61).