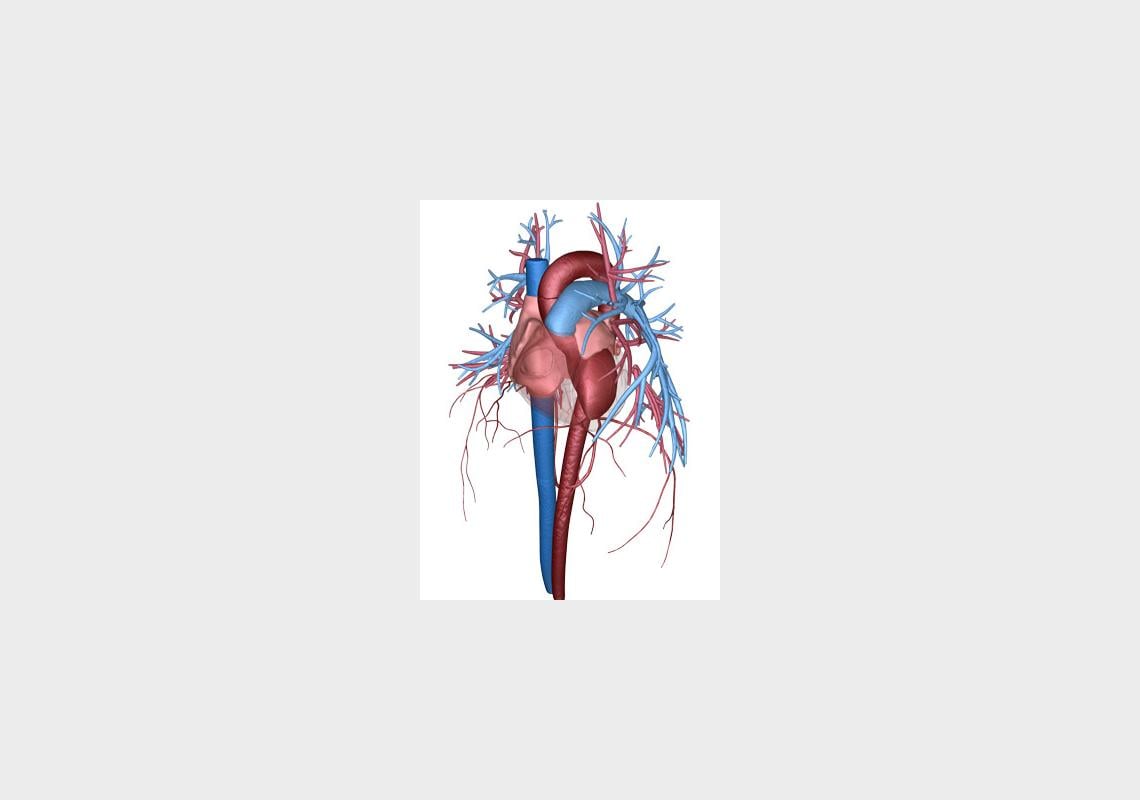
The heart circulatory system — the left ventricle is the dark red chamber shown on the right side of the image. © 2015 A*STAR Institute of High Performance Computing
Quantifying the damage caused to specific parts of the heart by cardiac arrest is key to providing effective treatment and accurate prognoses for millions of people worldwide. Now, Singapore's Agency for Science, Technology and Research (A*STAR) researchers have developed a computational method that uses magnetic resonance imaging data to assess the extent of damage to the left ventricle, the heart’s powerhouse [1].
Heart attacks — medically known as myocardial infarctions — damage the heart tissue, leading to scarring, impaired function and sometimes death. “Heart attacks are very complicated,” explains Teo Soo-Kng of the A*STAR Institute of High Performance Computing, “but in general, when the left ventricle is affected it usually leads to worse outcomes for the patient as that is the largest and most powerful pump in the heart.”
After an infarction, clinicians need an accurate measure of the damaged sustained to the left ventricle to decide on the best possible treatment. Clinical assessments based on changes in the amount of ejected blood during a heartbeat, called the ‘ejection fraction’, do not provide enough region-specific information about the damaged tissue. On the other hand, cardiac magnetic resonance imaging combined with a contrast agent is able to measure tissue scarring and provide region-specific information. However, such an approach only provides a semi-quantitative measure of the damage incurred.
Teo and his A*STAR colleagues decided to combine the best of both worlds to develop a robust, quantitative and region-specific measure that could be clinically useful. Using magnetic resonance imaging scans from clinical partners the team constructed a three-dimensional model of the left ventricle. They then used this to compute the ‘regional’ ejection fraction and the change in left ventricle surface area during a heartbeat — or ‘regional area strain’. These measures enable clinicians to quickly pinpoint which segments of the left ventricle have been compromised by a heart attack.
“Our method is not just useful for diagnostics, but also potentially for monitoring,” Teo remarks. “It could ultimately be used for clinicians to assess how well the patient responds to treatment — for example, whether the degeneration of the heart function stabilizes or continues.” This is reflected by the team’s ongoing trials with clinical partners, focused on relapse and heart failure.
Teo and the team are also implementing a server infrastructure for the technique that would increase its usefulness for time-sensitive clinical applications. “We are trying to reduce the time frame. At the moment it can range from days to a week — but we’re aiming to get it down to around half an hour or even less,” he says.
The A*STAR-affiliated researchers contributing to this research are from the Institute of High Performance Computing. More information about the team’s research can be found at the Geometrical Modelling webpage.
Reference
[1] Teo, S.-K., Vos, F. J. A., Tan, R.-U., Zhong, L. & Su, Y. Regional ejection fraction and regional area strain for left ventricular function assessment in male patients after first-time myocardial infarction. Journal of the Royal Society Interface 12, 20150006 (2015).
The Geometrical Modelling group. Seated left to right: Yang Xu Lei, Su Yi (senior author of the paper), Calvin Lim Chi Wan; standing left to right: Wong Sum Thai, Yeo Si Yong, Tan May Ling, Senthil Kumar Selvaraj, Like Gobeawan; absent with apologies: Teo Soo Kng. © 2015 A*STAR Institute of High Performance Computing