Image 1: Front row (seated, left to right): IBN researchers Dr Karthikeyan Kandasamy and Ms Jacqueline Chuah. Second row (left to right): Mr Peng Huang and Dr Sijing Xiong from IBN, Dr Ran Su of BII and IBN’s Dr Daniele Zink. Third row (left to right): IBN’s Mr Kim Guan Eng and Dr Yao Li, with BII’s Dr Lit-Hsin Loo. Copyright: IBN A*STAR
Singapore, October 9, 2015 – Researchers from the Institute of Bioengineering and Nanotechnology (IBN) have developed the first animal-free screening platforms capable of predicting the toxic effects of compounds on the human kidney accurately. Their latest technological advancement involves the use of human induced pluripotent stem cells (iPSCs) in their renal screening platform (Kandasamy et al., 2015). To realize this breakthrough, the scientists have developed an effective way of producing human renal cells from iPSCs, and have combined this with machine learning methods that improved the automated and accurate prediction of nephrotoxicity. In addition to predicting toxicity, the novel iPSC-based platform also correctly identifies injury mechanisms, which can help to advance understanding of the tested compounds.
IBN Executive Director Professor Jackie Y. Ying says, “Our new kidney screening platforms will be very useful for many industries that require a reliable tool for evaluating the safety of compounds and ingredients. For example, the chemical and pharmaceutical industries produce a large number of new compounds that need to be screened and tested. Likewise, there is a demand in the food and consumer care industries for efficient lab tools to predict the safety of novel ingredients in their products.”
Due to their role in the elimination of drugs and other foreign compounds from the body, the kidneys are a main target for compound-induced toxicity. Many widely used chemicals and drugs, such as anti-cancer drugs, antibiotics and immunosuppressants, are harmful to the kidneys and may cause organ damage or failure. Indeed, approximately 20% of hospital and community acquired cases of acute kidney injury were caused by such compounds (Tiong et al., 2014). This poses serious problems to patients and doctors. Further, a drug’s toxic effect on the kidney would typically be discovered only in the late stages of drug development or even after the product has been marketed. Therefore, the ability to determine a new drug’s toxicity earlier in the development phase would be of great interest to pharmaceutical companies, which could spend approximately USD 1-2 billion on average to develop a new drug (Segall and Barber, 2014).
Among the many challenges associated with using animal models to predict the nephrotoxicity of a new compound are the long time required, high costs involved, unreliable results due to inter-species differences, as well as ethical issues. Therefore, researchers have focused their efforts on developing cell-based screening methods. Animal-free screening methods are now also mandatory for cosmetic companies selling their products in the EU, India and Israel after the implementation of animal testing bans in these countries. Validated and accepted animal-free methods for predicting nephrotoxicity are currently not available in the market.
Over the past three and a half years, a research team led by IBN Team Leader and Principal Research Scientist Dr Daniele Zink has developed the first and only cell-based renal screening platforms that can predict nephrotoxicity in humans with high accuracy. Earlier versions of these platforms were based on human primary renal proximal tubular cells (Li et al, 2013) or similar cells derived from human embryonic stem cells or hESCs (Li et al, 2014). However, primary cells that are directly harvested from the human body are associated with various problems, such as limited availability, while the use of hESCs may give rise to ethical and legal concerns. Therefore, the researchers worked on an approach using human iPSCs. As iPSCs can be generated from cells that are easily available in any person (e.g. cells growing on the body surface), they could also be used to develop patient- and disease-specific models. Such models would help to provide a better understanding of renal disease and facilitate the development of personalized therapies and drugs.
According to Dr Zink, “We have developed the fastest and most efficient protocol for generating kidney cells from induced pluripotent stem cells. Within eight days, it yielded highly pure kidney cells that were suitable for compound screening. We also worked closely with Dr Lit-Hsin Loo’s team from the Bioinformatics Institute, which developed the data analysis procedures and machine learning methods that allow us to predict drug-induced nephrotoxicity with great accuracy. We were further able to identify injury mechanisms and drug-induced cellular pathways by using automated cellular imaging. We hope that our work will contribute to the development of safer products in future.”
This research was supported by the A*STAR Joint Council Office. The researchers plan to work with industrial partners to further validate and apply their renal screening platforms.
END
References:
1. K. Kandasamy, J. K. C. Chuah, R. Su, P. Huang, K. G. Eng, S. Xiong, Y. Li, C. S. Chia, L.-H. Loo and D. Zink, “Prediction of Drug-Induced Nephrotoxicity and Injury Mechanisms with Human Induced Pluripotent Stem Cell-Derived Cells and Machine Learning Methods,” Scientific Reports, 5 (2015) 12337.
2. H. Y. Tiong, P. Huang, S. Xiong, Y. Li, A. Vathsala and D. Zink, “Drug-Induced Nephrotoxicity: Clinical Impact and Pre-Clinical In Vitro Models,” Molecular Pharmaceutics, 11 (2014) 1933-1948.
3. M. D. Segall and C. Barber, “Addressing Toxicity Risk When Designing and Selecting Compounds in Early Drug Discovery,” Drug Discovery Today, 19 (2014) 688-693.
4. Y. Li, Z. Y. Oo, S. Y. Chang, P. Huang, K. G. Eng, J. L. Zeng, A. J. Kaestli, B. Gopalan, K. Kandasamy, F. Tasnim and D. Zink, “An In Vitro Method for the Prediction of Renal Proximal Tubular Toxicity in Humans,” Toxicology Research, 2 (2013) 352-362.
5. Y. Li, K. Kandasamy, J. K. Chuah, Y. N. Lam, W. S. Toh, Z. Y. Oo and D. Zink, “Identification of Nephrotoxic Compounds with Embryonic Stem Cell-Derived Human Renal Proximal Tubular-Like Cells,” Molecular Pharmaceutics, 11 (2014) 1982-1990.
Media Contacts:
Elena Tan Nidyah Sani
Phone: +65 6824 7032 Phone: +65 6824 7005
Email: [email protected] Email: [email protected]
About the Institute of Bioengineering and Nanotechnology
Established in 2003, the Institute of Bioengineering and Nanotechnology (IBN) is the world’s first bioengineering and nanotechnology research institute. IBN’s mission is to conduct multidisciplinary research across science, engineering, and medicine for breakthroughs to improve healthcare and quality of life.
IBN’s research activities are focused in the following areas:
· Nanomedicine, where functionalized polymers, hydrogels and biologics are developed as therapeutics and carriers for the controlled release and targeted delivery of therapeutics to diseased cells and organs.
· Synthetic Biosystems, where biomimetic materials, innovative cell culture, 3D printing technologies, microfluidic systems and bioimaging are combined to develop novel approaches for regenerative medicine, in vitro compound screening, and disease modeling.
· Biodevices and Diagnostics, which involve nanotechnology and microfabricated platforms for high-throughput biomarker and drug screening, automated biologics synthesis, and rapid disease diagnosis.
· Green Chemistry and Energy, which encompass the green synthesis of chemicals and pharmaceuticals, catalytic conversion of biomass, utilization of carbon dioxide, and new nanocomposite materials for energy applications.
Scientific Impact
· More than 1,000 papers published in leading scientific journals
· Over 1,100 seminars and presentations at international conferences, including over 700 invited, keynote and plenary lectures
· Organized premier scientific meetings such as the International Conference on Bioengineering and Nanotechnology, Nano Today Conference, and the IBN International Symposium
Technological and Commercialization Impact
· 341 active patents and patent applications
· 84 licensed patents and patent applications
· 9 spin-off companies
· 153 active research collaborations with industrial, clinical and academic partners
Nurturing Future Research Talents
· Trained 116 PhD students
· More than 90,300 students and teachers from 290 local and overseas schools/universities have participated in IBN’s Youth Research Program
· Over 2,200 students and teachers have completed research attachments at IBN
For more information on IBN, please visit www.ibn.a-star.edu.sg.
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that spearheads economic oriented research to advance scientific discovery and develop innovative technology. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit society.
As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by contributing to societal benefits such as improving outcomes in healthcare, urban living, and sustainability.
We play a key role in nurturing and developing a diversity of talent and leaders in our Agency and Research Institutes, the wider research community and industry. A*STAR oversees 18 biomedical sciences and physical sciences and engineering research entities primarily located in Biopolis and Fusionopolis.
For more information on A*STAR, please visit www.a-star.edu.sg.
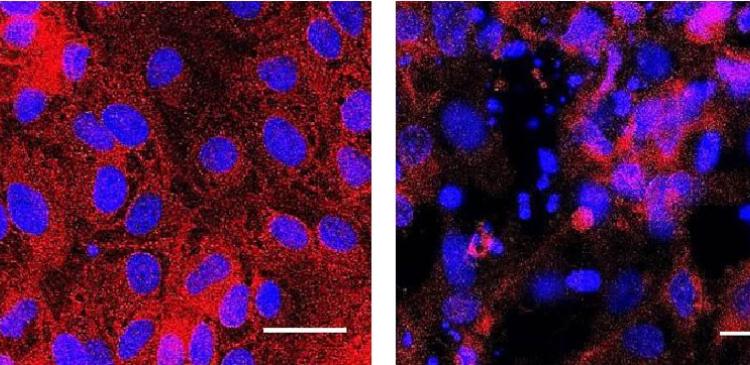
Image 2: Renal proximal tubule-like cells (nucleus: blue, cytoplasm: red) derived from induced pluripotent stem cells that have been left untreated (left) or treated with aristolochic acid (right), a herbal nephrotoxic compound widely used in Traditional Chinese Medicine and other herbal remedies, which is now banned in many countries. Scale bar = 25 μm. Copyright: IBN A*STAR