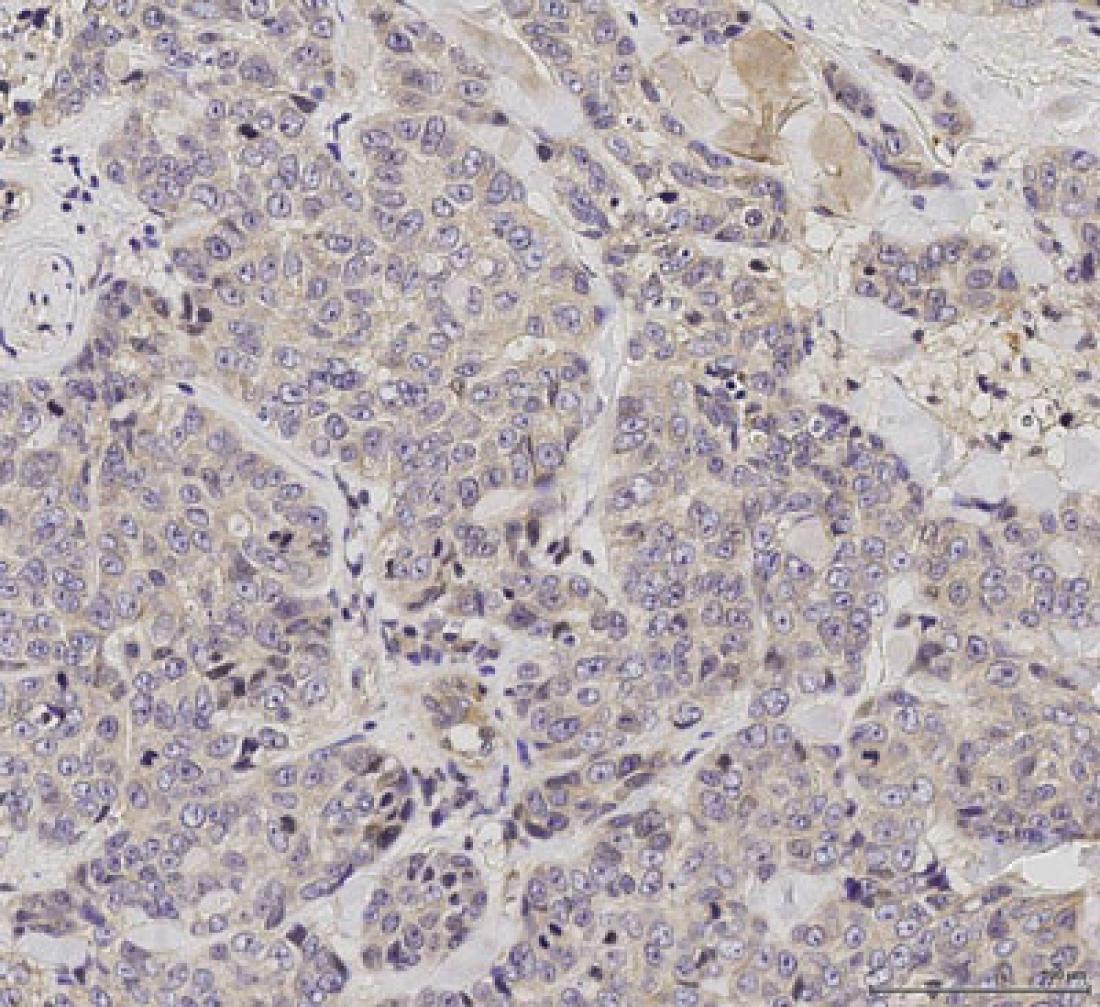
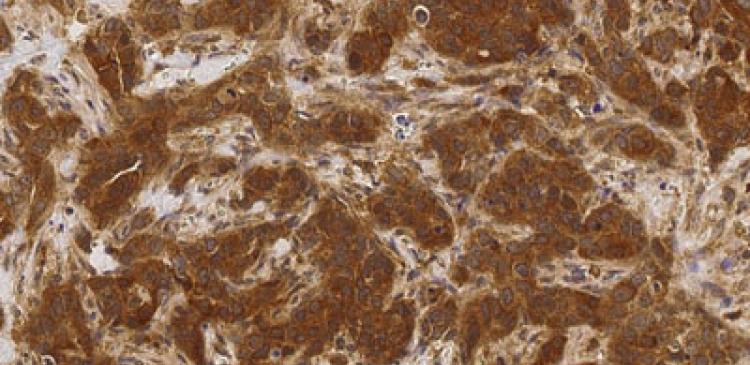
Primary tumour. The images show a higher level of phosphorylated IRAK1 in recurrent tumour compared to that in the original primary tumour.
Singapore—Scientists at the Bioinformatics Institute (BII) and Genome Institute of Singapore (GIS), research institutes under the Agency for Science, Technology and Research (A*STAR), have made discoveries that could lead to new ways of diagnosing and treating breast cancer. The scientists from both institutes used large-scale genomic data of breast cancers, demonstrating the use of computational techniques to increase understanding of diseases and improve patient treatments.
Breast cancer is the most frequent cancer diagnosed amongst women, with an estimated 1.67 million new cancer cases worldwide in 2012[1]. In Singapore, more than 9,000 women were diagnosed with breast cancer between 2010 and 2014[2]. Breast cancer is also the leading cause of cancer death in females locally.
Improving classification and treatment of prevalent type breast cancer
The BII scientists identified and characterised two new major classes of invasive ductal carcinoma (IDC), which comprise about 80% of all breast cancers[3]. The increased knowledge of these genetically and clinically distinct classes will improve the diagnosis, prognosis and treatment of IDC, paving the way for personalised treatment with better patient outcomes (See Annex A).
Employing integrative bioinformatics and analyses, the scientists found that the intermediate grade (histologic grade 2) - assigned to approximately 50% of IDC cases - did not exist at the molecular level. IDC tumours in this grade would be better classified under the two major classes described, to enable better prediction of disease outcomes and for optimal treatment to be assigned.
Identifying new targets for treatment of aggressive breast cancer
In a separate study, scientists from GIS discovered a molecular mechanism that can be targeted to treat a more aggressive type of breast cancer, called triple-negative breast cancer (TNBC). The researchers identified a crucial protein whose increased activity promotes breast cancer metastasis and resistance to paclitaxel, a first line chemotherapeutic agent to treat breast cancer.
Additionally, treating this pathway may also contribute to the prevention of tumour recurrence, which is the main reason for breast cancer patient mortality.
While increased awareness of the need for early detection has led to improved survival in breast cancer patients, the development of improved treatment strategies remains important to further reduce mortality rates.
Dr Benjamin Seet, Executive Director of A*STAR’s Biomedical Research Council, said, “These findings advance our understanding of breast cancer, which is the most common cancer affecting women in Singapore. More importantly, it allows us to develop precise and effective treatment strategies for these particular types of breast cancer, as well as to discover new drugs for the patients who do not get better.”
Notes to Editor:
The research findings described in this media release can be found in the journals:
1. Nature Communication, under the title, “IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel" by Zhen Ning Wee1, Siti Maryam J.M. Yatim1, Vera K. Kohlbauer1, Min Feng1, Jian Yuan Goh1, Bao Yi1, Puay Leng Lee1, Songjing Zhang1, Pan PanWang2,3, Elgene Lim4,Wai Leong Tam1,5, Yu Cai3,6, Henrik J. Ditzel7,8, Dave S.B. Hoon9, Ern Yu Tan10 & Qiang Yu1,3,11,12.
1 Cancer Therapeutics and Stratified Oncology, Genome Institute of Singapore, A*STAR (Agency for Science, Technology and Research), 60 Biopolis Street, 02-01, Biopolis 138672, Singapore.
2 First Affiliated Hospital, Jinan University, Guangzhou 510632, China.
3 Cancer Research Institute, Jinan University, Guangzhou 510632, China.
4 The Kinghorn Cancer Center, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, Sydney, New SouthWales 2010, Australia.
5 Cancer Science Institute, National University of Singapore, Singapore 117599, Singapore.
6 School of Pharmacy, Jinan University, Guangzhou 510632, China.
7 Department of Cancer and Inflammation Research, Institute of Molecular Medicine, University of Southern Denmark, Odense 5000, Denmark.
8 Department of Oncology, Odense University Hospital, Odense 5000, Denmark.
9 Department of Molecular Oncology, John Wayne Cancer Institute, Santa Monica, California 90404, USA.
10 Department of General Surgery, Tan Tock Seng Hospital, Singapore 308433, Singapore.
11 Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
12 Cancer and Stem Cell Biology, DUKE-NUS Graduate Medical School of Singapore, Singapore 169857, Singapore.
Correspondence and requests for materials should be addressed to Q.Y.
(email: [email protected]).
2. Oncotarget, under the title, “Genome and transcriptome delineation of two major oncogenic pathways governing invasive ductal breast cancer development” by Luayy Aswad1,2, Surya Pavan Yenamandra1, Ow Ghim Siong1, Oleg Grinchuk1, Anna V. Ivshina1, Vladimir A. Kuznetsov1,2
1Bioinformatics Institute, Agency for Science, Technology and Research (A*STAR)
2School of Computer Engineering, Nanyang Technological University (NTU)
Correspondence should be addressed to Vladimir A Kuznetsov, Genome and Gene Expression Data Analysis Division, A*STAR Bioinformatics Institute, 30 Biopolis Street, #07-01, Singapore 138671. E-mail: [email protected]
Full text of the paper can be accessed online from: http://www.impactjournals.com/oncotarget/index.php?journal=oncotarget&pa...
_______________________________________________________________
Enclosed:
Annex A – Findings by A*STAR’s BII pave way for major shift in classification and treatment of breast cancer
Annex B – A*STAR’s GIS finds new therapeutic target for aggressive breast cancer
For media queries and clarifications, please contact:
Vanessa Loh (Ms)
Senior Officer, Corporate Communications
Agency for Science, Technology and Research
Tel: +65 6826 6395
Email: [email protected]
Winnie Lim (Ms)
Head, Office of Corporate Communications
Genome Institute of Singapore, A*STAR
Tel: +65 6808 8013
Email: [email protected]
________________________________________________________________
About the Bioinformatics Institute (BII)
The Bioinformatics Institute (BII) is an institute of the Agency for Science, Technology and Research (A*STAR). BII was set up in July 2001 as part of the national initiative to foster and advance biomedical research and human capital for a vibrant knowledge-based Singapore. With a multi-disciplinary focus and collaborative outlook, BII recognises the need for depth and breadth in all its activities for building a thriving world-class biomedical research, graduate training and development hub in Singapore. In addition, BII is proactively involved in building a national resource centre in bioinformatics to meet the evolving needs of the scientific community in Singapore.
For more information on BII, please visit: www.bii.a-star.edu.sg
About the Genome Institute of Singapore (GIS)
The Genome Institute of Singapore (GIS) is an institute of the Agency for Science, Technology and Research (A*STAR). It has a global vision that seeks to use genomic sciences to achieve extraordinary improvements in human health and public prosperity. Established in 2000 as a centre for genomic discovery, the GIS will pursue the integration of technology, genetics and biology towards academic, economic and societal impact.
The key research areas at the GIS include Human Genetics, Infectious Diseases, Cancer Therapeutics and Stratified Oncology, Stem Cell and Regenerative Biology, Cancer Stem Cell Biology, Computational and Systems Biology, and Translational Research.
The genomics infrastructure at the GIS is utilised to train new scientific talent, to function as a bridge for academic and industrial research, and to explore scientific questions of high impact.
For more information about GIS, please visit www.gis.a-star.edu.sg.
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that spearheads economic oriented research to advance scientific discovery and develop innovative technology. Through open innovation, we collaborate with our partners in both the public and private sectors to benefit society.
As a Science and Technology Organisation, A*STAR bridges the gap between academia and industry. Our research creates economic growth and jobs for Singapore, and enhances lives by contributing to societal benefits such as improving outcomes in healthcare, urban living, and sustainability.
We play a key role in nurturing and developing a diversity of talent and leaders in our Agency and Research Institutes, the wider research community and industry. A*STAR oversees 18 biomedical sciences and physical sciences and engineering research entities primarily located in Biopolis and Fusionopolis.
For more information on A*STAR, please visit www.a-star.edu.sg.
ANNEX A
ANNEX A – FINDINGS BY A*STAR’S BII PAVE WAY FOR MAJOR SHIFT IN CLASSIFICATION AND TREATMENT OF BREAST CANCER
Invasive Ductal Carcinoma (IDC), as with many other malignant tumours, is very heterogeneous[4]. Histological grading, which entails the microscopic study of cellular structure and function, classifies tumours into three grades which reflect the aggressiveness of tumours. This method is widely adopted by oncologists as a factor for prognosis and assignment of therapy. However, for intermediate grade (Grade 2) tumours, this method of evaluation is highly subjective, with as little as 50% agreement between observers in some cases[5] and little understanding of its molecular basis.
Using integrative bioinformatics and analyses of data from more than 1,200 patients, the BII scientists found that a 22-gene panel could be used to classify IDC cases into two new major classes that reflect the aggressiveness of the tumours, namely Low Genetic Grade (LGG) and High Genetic Grade (HGG). Cancers in each of these classes demonstrated distinct genomic alterations and differences in the way they develop, marking a shift in understanding by suggesting that IDC does not necessarily follow a gradual progressive model of disease.
Dr Lee Soo Chin, Senior Principal Investigator at the Cancer Science Institute of Singapore and Senior Consultant at the National University Cancer Institute, Singapore (NCIS), commented on the significance of these findings, “While Grade 1 and Grade 3 tumours clearly represent tumours belonging to two ends of a spectrum with distinctly different aggressiveness, the clinical relevance of a Grade 2 tumour is less certain, making the information less useful when making therapeutic decisions in the clinic. In this study, investigators from BII classified breast cancers into two new genetic subclasses, removing the intermediate group of histological Grade 2, which have distinctly different survival and different genetic aberrations, and can be a useful classification to use in the clinic when validated.”
The 22-gene panel could thus be used to complement or replace the histological grading prediction system, improving clinical practice with better molecular characterisation of tumours in either class. The 22- gene panel, together with other genetic and functional features of these two IDC molecular classes identified by the BII team, could serve as novel prognostic and therapeutic biomarkers that could lead to the development of personalised systemic therapies using drugs that are already available.
Dr Vladimir Kuznetsov, Senior Principal Investigator at BII who led the study said, “These findings demonstrate the continuing importance of basic sciences and specifically, integrative genomic studies in understanding the molecular basis of cancer aggressiveness to improve treatment of cancer patients. We hope that the global research community can harness the power of big genomic data analysis in innovative ways to improve healthcare.”
ANNEX B
ANNEX B – A*STAR’S GIS FINDS NEW THERAPEUTIC TARGET FOR AGGRESSIVE BREAST CANCER
Researchers from GIS identified a new protein involved in metastatic progression and development of drug resistance in breast cancer, which could be a target for new treatments for triple negative breast (TNBC). The study, published in the journal Nature Communication, was done in collaboration with researchers from Tan Tock Seng Hospital in Singapore, John Wayne Cancer Institute in California, University of Southern Denmark, and Jinan University in China.
Patients with TNBC have limited treatment options because their tumour cells lack the three receptors—estrogen, progesterone and human epidermal growth factor receptor 2 (HER-2) —commonly targeted in hormone or chemotherapy. The primary features of TNBC include early metastatic relapse and poor outcomes. Currently, chemotherapy remains the main therapeutic option. However, this method often does not work well, and even when effective in the beginning, stops working after a while due to development of resistance to the treatment.
The study was led by Dr Qiang Yu, a senior group leader and professor at the GIS, whose laboratory focuses on investigating how cancer cells develop resistance to drug treatments; and first author Dr Zhen Ning Wee, a postdoctoral fellow in Dr Yu’s laboratory. Through interrogating large-scale genomic database of breast cancers, Dr Yu’s team set out to find an “upstream” regulator of the NF-kB pathway, which is well known to be involved in cancer progression and drug resistance. They identified a protein kinase, called interleukin-1 receptor-associated kinase 1 (IRAK1), which transmits signals to NF-kB to produce inflammatory cytokines in breast cancer cells. They found that TNBC has a higher expression of IRAK1 compared to other types of breast cancer.
More significantly, the team found that IRAK1 becomes more active when breast cancer cells become metastatic and develop resistance to paclitaxel. They further demonstrated that inhibiting IRAK1 can reduce metastatic progression and overcome paclitaxel resistance. Specifically, the team showed that an IRAK1 inhibitor can greatly sensitise the ability of paclitaxel to commit programmed suicide, or apoptosis, which is lost when cancer cells develop resistance.
“Our results suggest that IRAK1 has a crucial role in the development of drug resistance. We can therefore speculate that upfront administration of drugs that can block IRAK1 activity is highly likely to prevent tumour recurrence. This is particularly worthy of further investigation, given that breast cancer mortality is mainly caused by tumour recurrence due to failed chemotherapy,” Dr Yu said.
“Our long-term goal is to develop drugs that target IRAK1 for a novel combination approach, to efficiently trigger apoptosis in recurrent metastatic cancer cells. By achieving this, we will be able to generate therapeutic effects by eliminating metastatic burden and overcome drug resistance,” added Dr Yu.
GIS Executive Director Prof Huck Hui Ng said, "Research efforts in close collaboration with clinical communities to address clinical problems are crucial towards successful translational research. The findings in this study provide new mechanistic insights into breast cancer progression, which paves the way for developing a more precise treatment strategy for refractory breast cancer patients.”
[1] International Agency for Research on Cancer, “GLOBOCAN 2012 Fact Sheet” http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
[2] Singapore Cancer Registry, Interim Annual Report Trends in Cancer Incidence in Singapore 2010 – 2014, https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library...
[3] BreastCancer.org, http://www.breastcancer.org/symptoms/types/idc
[4] Tumour heterogeneity refers to the differences between tumours of the same type within patients, and between cancer cells within a tumour. Such diversity makes it challenging to classify the tumors and design effective treatment strategies.
[5] American Cancer Society, CA Cancer J Clin. 2015 Jan-Feb;65(1):5-29.