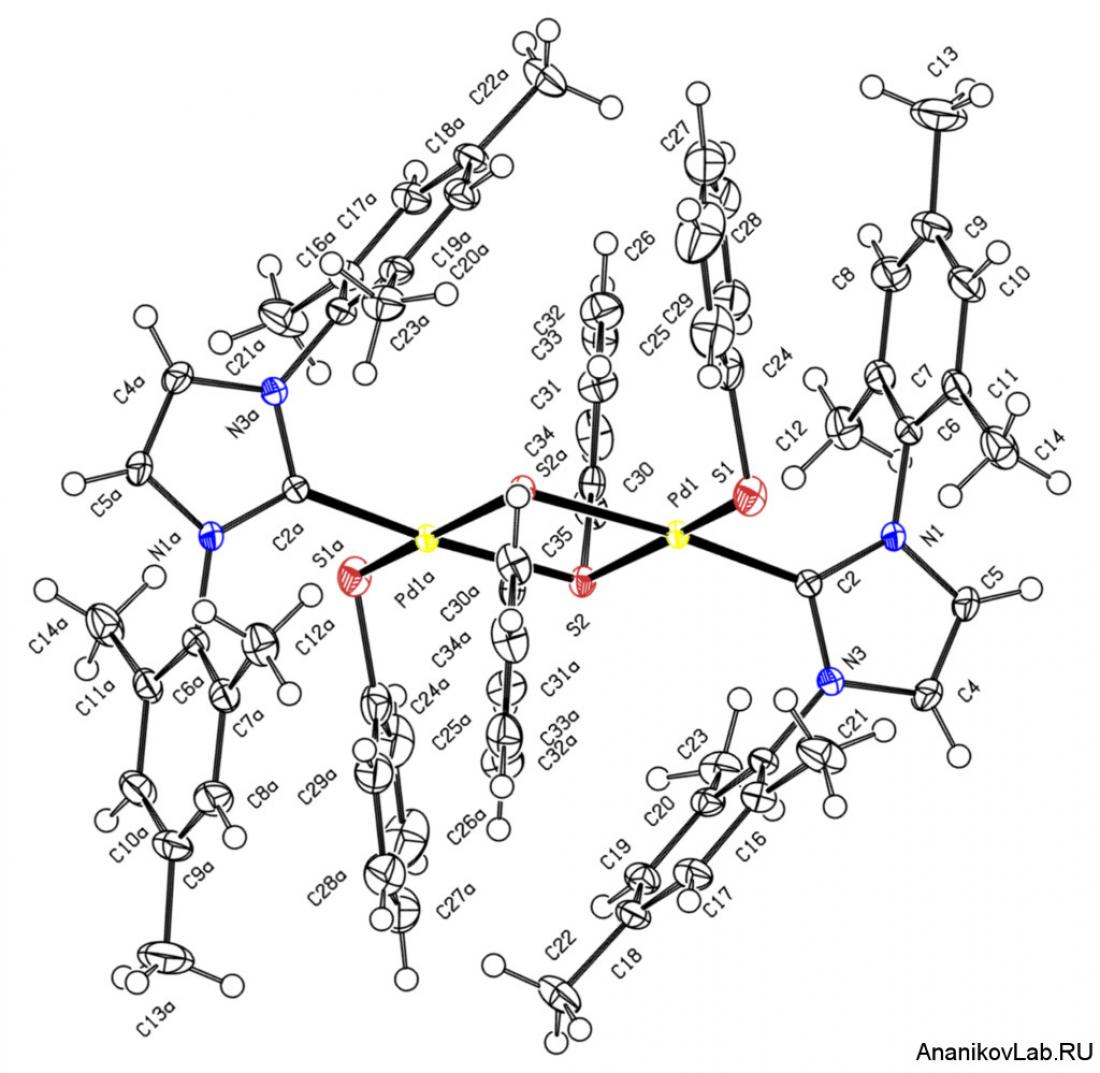
A unique four-membered Pd2S2 ring created in the catalytic chemistry.
Mercaptans or thiols are a special class of organic compounds that contains sulfur functional group, RSH. Various sulfur compounds are highly demanded in the formation of new materials in photonics, optics, pharmaceutical industry, organic chemistry, and nanotechnology.
Sulfur derivatives are, by far, the richest fossil source of functional molecules available in nature. Indeed, a diversity of sulfur species is present as contaminants in crude oil. Unfortunately, there are still no efficient technological tools to separate sulfur compounds from crude oil and utilize them in materials production. Petroleum industry wastes billions of tones of valuable compounds, which are annually destroyed to elemental sulfur.
It is a well-known fact that humans are very sensitive to thiols. Small molecular thiols have an extremely unpleasant smell, which even in trace-level concentration (1-5 parts per billion) can be easily detected by human’s nose.
A unique palladium catalyst was developed in the laboratory of Prof. Ananikov at Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences. Pd complex with NHC ligand furnished chemical transformations of thiols into vinyl monomers, a useful component of new generation of polymeric materials. Even challenging EtSH and PrSH thiols were involved in the reaction and produced excellent outcome.
Chemical transformation was performed using atom–economic approach, which assures high yield and complete selectivity. This means that a pure product can be obtained just after completion of the reaction and isolation of the catalyst.
Mechanistic studies have revealed the key role of nuclearity of transition metal complexes (Figure 2) in the catalytic cycle. Monometallic Pd complex mediated quick reaction, where bimetallic Pd complex reacted much slower. The mechanistic findings are connected to the catalyst evolution problem and to the role of nucleation to nanoparticles revealed by this group earlier (doi: 10.1021/jo402038p).
Upon addition to alkynes, thiols were efficiently converted to vinyl thioethers – stable monomenrs, which are easy to handle and do not have an unpleasant odour.
Here comes the logical solution to many chemical dilemmas: a right catalyst may turn even unpleasant chemicals into valuable and friendly products.
The article «Pd-NHC Catalytic System for the Efficient Atom-Economic Synthesis of Vinyl Sulfides from Tertiary, Secondary, or Primary Thiols» by Evgeniya Degtyareva, Julia Burykina, Artem Fakhrutdinov, Evgeniy Gordeev, Victor Khrustalev, and Valentine Ananikov was published in ACS Catalysis journal published by American Chemical Society.
Reference: ACS Catal. 2015, 5, 7208−7213; DOI: 10.1021/acscatal.5b01815
On-line link: http://dx.doi.org/10.1021/acscatal.5b01815