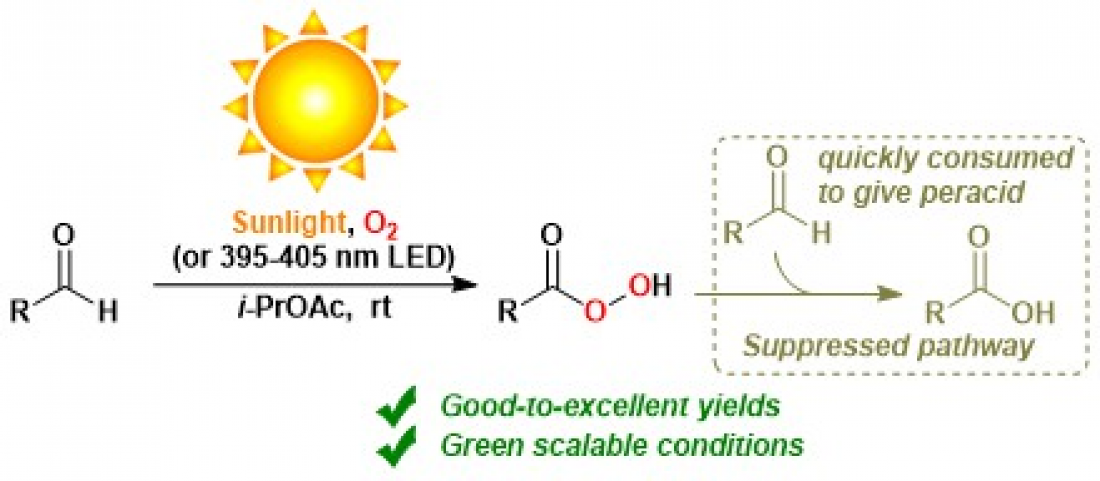
Fig. Light-induced autoxidation of aldehydes to peracids
Researchers from Osaka University and collaborating partners have deduced the factors that underpin common oxidation of aldehydes in air, which has helped optimize the environmental sustainability of a wasteful or even dangerous chemical synthesis
Osaka, Japan – Approximately 5% of global carbon emissions are attributable to producing the chemicals that are essential to modern life. Creating a sustainable solution to one chemical reaction in particular – the autoxidation of aldehydes – has challenged researchers for decades.
Now, in a study recently published in Green Chemistry, researchers from Osaka University, Shizuoka Institute of Science & Technology, and collaborating partners have solved this problem. Through reaction kinetics and mathematical modelling, they studied the key details of autoxidation of aldehydes in various solvents, atmospheric conditions, and light sources. The identified environmentally friendly reaction conditions might serve as inspiration for improving other industrially common chemical syntheses.
Autoxidation simply refers to oxidation in air at typical temperatures, without a flame or spark input. For example, autoxidation of a common molecule known as an aldehyde produces a molecule known as a peracid. Peracids are incredibly useful because they can catalyzed the conversion of a wide range of molecules by well-established chemistry. Unfortunately, the reaction conditions that are commonly used to produce peracids are wasteful, require dangerous additives, or have other fundamental complications. Optimizing the sustainability of the autoxidation of aldehydes was the goal of the research team’s study.
“In our work, we comprehensively study the reaction chemistry that underpins autoxidation of aldehydes into peracids or carboxylic acids,” explains Mohamed Salem, lead author of the study. “The experimental setup is simple and suitable for gram-scale syntheses.”
The researchers report 18 examples of fast reactions of various aldehydes into peracids, in a pure oxygen atmosphere. These conditions suppressed continued reaction into carboxylic acids. They furthermore report 32 examples of slow reactions of various aldehydes into carboxylic acids through peracid intermediates, in a normal atmosphere. Some of these 50 reactions proceeded best under sunlight, or alternatively under an LED light. All reactions were at approximately room temperature, and in safe solvents. This work is the first time that peracids were synthesized from aldehydes using only sunlight and oxygen.
“We’re excited because our research solves a long-standing peracid synthesis problem in green chemistry,” says Shinobu Takizawa and Masayuki Kirihara, senior authors. “Our comprehensive studies will help researchers understand the unique chemistry that’s imparted by different functional groups in the starting material.”
This work is an important step forward in solving a previously vexing problem in peracid synthesis that has limited the environmental sustainability of producing such molecules. The optimized autoxidation reaction conditions identified in this study are applicable to a wide range of common chemical starting materials. Because the procedures are safe and cheap, practical applications in diverse syntheses should be straightforward. The research team is currently strengthening its collaboration with MANAC Chemical Partners Co., Ltd. to commercialize these research outcomes.
###
The article, “Light-induced autoxidation of aldehydes to peracids and carboxylic acids,” was published in Green Chemistry at DOI: 10.1039/d3gc02951d
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en
About Shizuoka Institute of Science & Technology
Shizuoka Institute of Science and Technology (SIST) was established on April 4, 1991 in Fukuroi city, Shizuoka prefecture in Japan. The university has an enrolment of approximately 1500 students in two faculties: the Faculty of Science and Technology and the Faculty of Informatics.
Website: https://www.sist.ac.jp/en.html