□ The research team led by Professor Jong-Sung Yu of the Department of Energy Science and Engineering at DGIST (President Kook Yang) has developed a low-temperature method to synthesize a highly graphitized[1] carbon support[2] that will greatly improve the lifespan of hydrogen fuel-cells[3]. They expect that the results of this study will greatly increase the possibility of commercialization by being used in fuel cells for vehicles, batteries for water electrolysis, and drones.
□ The importance of hydrogen fuel-cells is increasing with the burgeoning need for eco-friendly energy. Therefore, studies to improve the performance and lifespan of hydrogen fuel-cells are underway, with particular focus on methods to increase the activity and utilization of platinum (Pt) as a fuel-cell catalyst.
□ Platinum-based fuel-cell catalysts are highly effective, but they are expensive and have poor long-term durability. To address this, the US Department of Energy is intensively seeking solutions to these issues to enable widespread use of fuel cells.
□ The principal issue with long-term durability is the instability of the platinum-based catalyst and its carbon support. High voltages generated during start-stop operations break down the carbon supports, causing the detachment of the supported platinum and a decrease in electrical conductivity. A solution to this reduction in fuel-cell performance is urgently needed, but as improving the durability of carbon materials is challenging, most research concentrates on platinum-based catalysts.
□ Increasing graphiticity enhances the durability of carbon materials. However, graphiticity can only be increased by high-temperature heat treatment (2,000 ℃ or higher) making it difficult to induce properties that can improve fuel-cell performance. Consequently, a new synthesis strategy is needed that can obtain useful properties of carbon materials in a less energy-intensive manner.
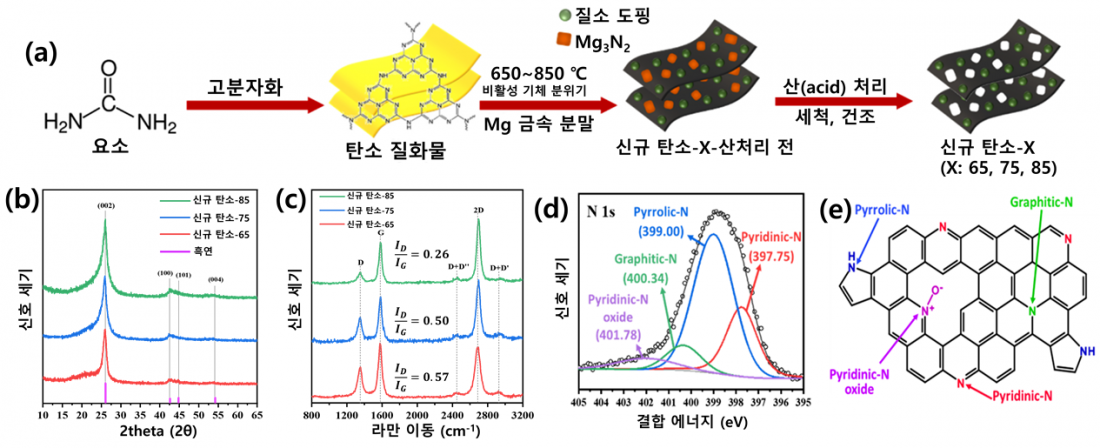
□ Professor Yu’s research team developed a new synthesis method that can introduce high graphiticity even at the relatively low temperature of 650 ℃. It is a simple process that heats a nitrogen-containing organic precursor with magnesium (Mg) powder. The temperature of the treatment was lowered in an unprecedented fashion by using the reducing power[4] of magnesium. In addition, magnesium acted as its own template[5] to provide a porous structure and a high specific surface-area to the highly graphitized carbon material. It was confirmed that sufficient nitrogen was doped into the carbon material by the low-temperature process.
□ The magnesium-based low-temperature treatment developed by the team produced an outstanding carbon support that met the three essential conditions of an electrochemical catalyst: graphiticity for stability and electric conductivity, excellent porosity and surface area, and heteroatom doping6
□ The research team expected that the high graphiticity would improve the durability of the carbon support and that the heteroatom doping would effectively prevent the dissolution of platinum by bonding stably with platinum nanoparticles. To verify this, they evaluated the durability of the platinum catalyst and the carbon support using the protocol proposed by the US Department of Energy.
□ Both the catalyst and the support showed stability that surpassed the targets of the US Department of Energy. The durability target for the carbon support was a 40% loss in activity per mass of platinum, but the platinum–highly graphitized nitrogen-doped carbon (Pt-HGNC) catalyst only suffered a 24% loss and was 3.5 times more stable than a platinum–carbon (Pt–C) catalyst.
□ Professor Yu of the Department of Energy Science and Engineering at DGIST said they “proposed a low-temperature synthesis strategy to overcome the limitations of existing carbon material production methods, and through this a new carbon support was synthesized. The newly developed fuel-cell catalyst to which it was applied is the first to satisfy the US Department of Energy’s durability standards for both the platinum catalyst and the carbon support”, and they “expect to contribute to the commercialization of fuel cells for vehicles and power generation and the improvement of the durability of fuel-cell catalysts.”
□ This study was jointly conducted by the team led by Professor Goddard at California Institute of Technology (Caltech), the team led by Professor Chang-Hyuck Choi at Pohang University of Science and Technology (Postech), and doctoral student Ha-Young Lee from the Department of Energy Science and Engineering at DGIST who participated as the first author. The results were published in Applied Catalysis B: Environmental (IF: 24.319), a top journal in the field of Environmental Science.
Correspondence Author E-mail : [email protected]
[1] Graphiticity: Properties similar to those of graphite, a chemically stable structure in which rings of six carbon atoms are connected to form layers.
[2] Carbon support: A commercially available carbon material for the dispersion of platinum nanoparticles, which is a catalyst for fuel cells.
[3] Hydrogen fuel cell: An eco-friendly power-generating technology that produces electricity using hydrogen and oxygen as fuel.
[4] Reducing power: The ability to lose electrons to (reduce) oxides.
[5] Template: A mold used to introduce a porous structure during carbon synthesis. Generally, inorganic substances, which are stable at high temperatures, are used and then removed after the heat treatment.