DGIST’s joint research team. From left are Professor Su-Il In and Professor Jong-Sung Yu.
DGIST’s joint research team led by Professor Jong-Sung Yu and Professor Su Il-in has developed a new titania photocatalyst that converts carbon dioxide into methane three times more efficiently than the existing photocatalyst by manipulating its surface.
Carbon dioxide is a major cause of global warming. Therefore, in order to control atmostpheric carbon dioxide concentration, many countries are actively working on numerous studies to investigate effective ways to transform carbon dioxide into chemical fuels such as methane, ethane and methanol. In particular, a high-efficiency photocatalyst is essential to help prevent the generation of secondary harmful substances when converting carbon dioxide into chemical fuels.
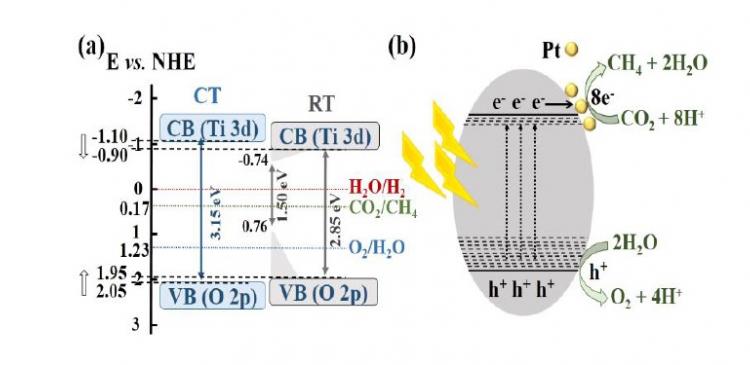
The research team has applied a simple magnesiothermic reduction method to synthesize oxygen-deficient titanium dioxide by removing oxygen atoms on the surface of titanium dioxide, which turns out to be a high-efficient photocatalyst that can effectively convert carbon dioxide into methane.
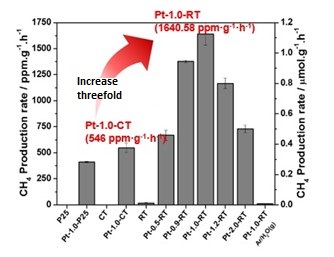
DGIST research team mentions that the newly developed photocatalyst illustrates controlled band gap by removing oxygen atoms on the surface of titanium dioxide through strong reduction of magnesium and hydrogen. This band gap control improves the light absorption and optimizes the efficient charge separation. As a result, the photocatalyst is found to increase conversion rate of carbon dioxide into methane up to threefold compared to the existing photocatalyst.
In addition, the study demonstrates that reduced titanium dioxide photocatalyst developed by DGIST team is superior to that of the existing titanium dioxide in terms of the conversion efficiency of carbon dioxide into methane. It also highlights the excellence of the current magnesiothermic reduction method which was applied for the preparation of reduced titanium dioxide photocatalyst through a relatively simple thermoreduction method with Mg metal and hydrogen gas.
Professor Su-Il In stated "The key of this study is that we have improved the efficiency of the existing titanium dioxide photocatalyst by using a relatively simple magnesiothermic reduction method." He added "By understanding the conversion mechanism of carbon dioxide into hydrocarbon, we expect to apply it to use carbon dioxide as resource in abatement technologies."
This study has been published online in May 8th issue of 'Applied Catalysis B: Environmental', an international academic journal of environmental catalyst.