Formate is a major carbon source for methanogenic archaea and a signature metabolite of enteric bacteria, such as Escherichia coli. Scientists have previously identified the integral membrane protein FocA as a formate transporter protein in E. coli. Nieng Yan and Yigong Shi at Tsinghua University in Beijing and co-workers1 have now determined the crystal structure of FocA. Their finding suggests that FocA may not be a transporter protein, but may instead be a channel protein.
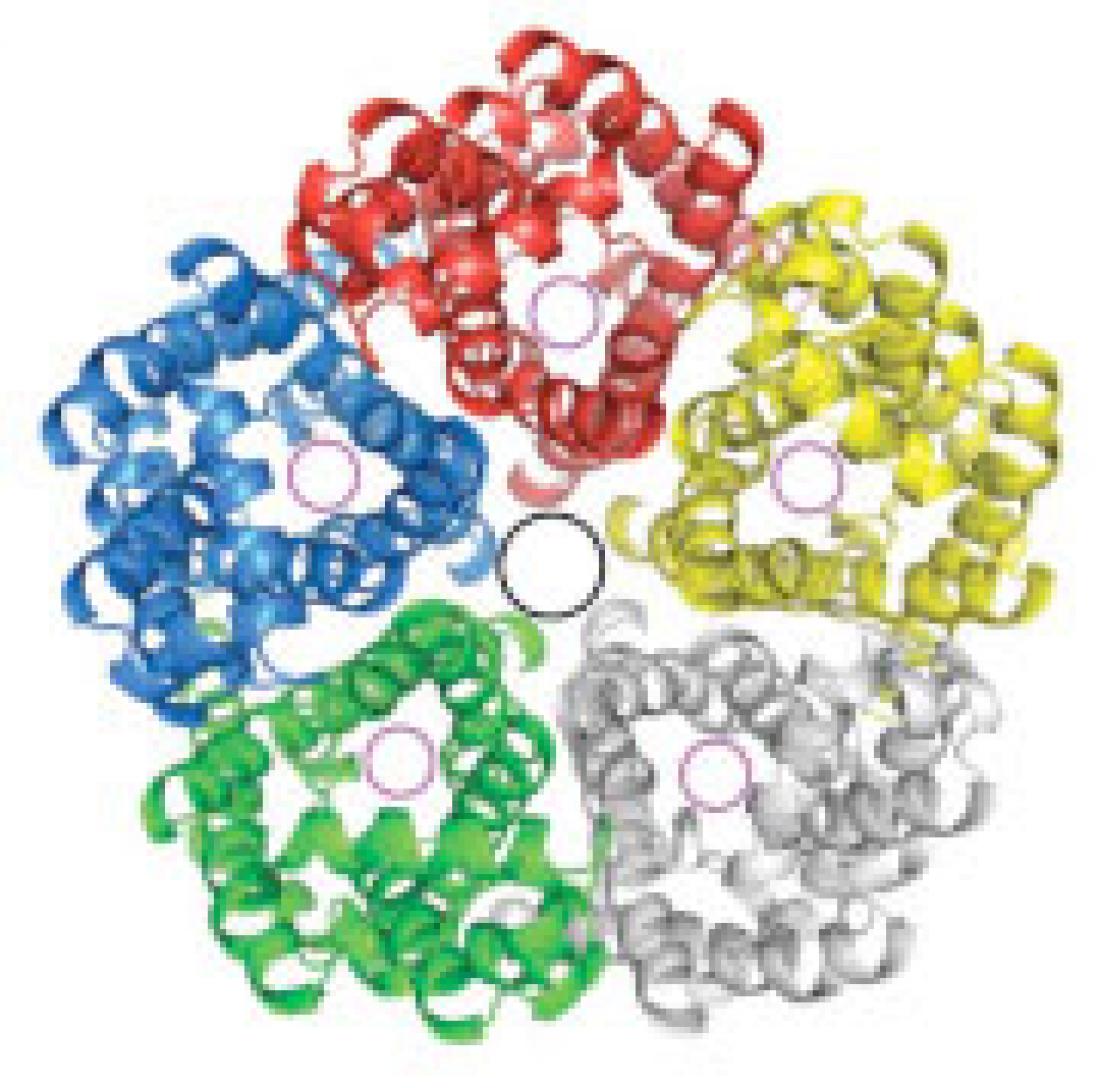
The researchers determined FocA to be a symmetric pentamer (see image) consisting of five identical subunits — or protomers — each with six transmembrane segments. Interestingly, FocA has a pore (black circle) in the centre of its pentameric assembly and an axial passage (magenta circle) in each of its protomers.
Despite a lack of sequence homology, the overall structure of the FocA protomer closely resembles that of aquaporin — the integral membrane protein that forms water channels in the membrane of biological cells. Unlike aquaporin, however, FocA conducts formate — instead of water molecules — in and out of the cell membrane.
The authors of this work are from:
Protein Science Laboratory, Ministry of Education, Tsinghua University, Beijing, China; State Key Laboratory of Biomembrane and Membrane Biotechnology, Center for Structural Biology, School of Life Sciences and School of Medicine, Tsinghua University, Beijing, China; Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, China.