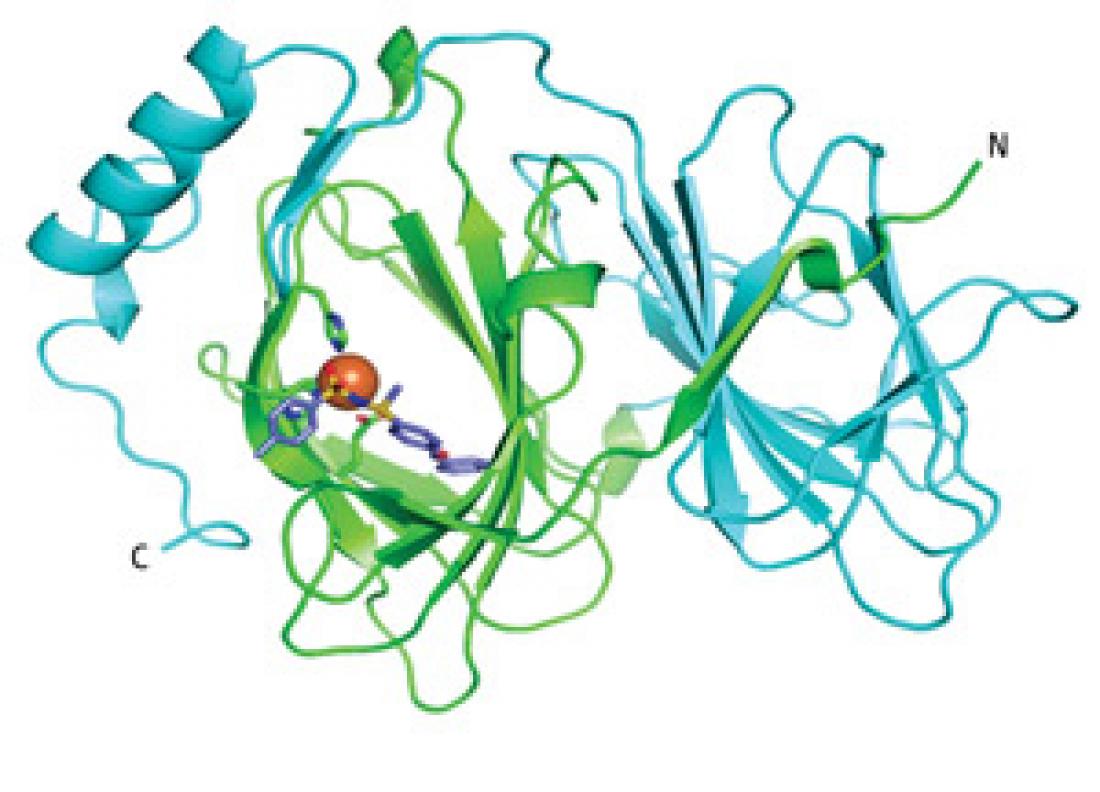
Figure 1: A structural diagram of the TPh A-pirin complex showing the small molecule (purple and red) stably lodged within a pocket of the protein (green and light blue).
As scientists continue to acquire immense amounts of genomic and biochemical data from various organisms, they routinely find themselves confronted by proteins of known structure but enigmatic function—and resolving those mysteries may require a chemical-based ‘fishing expedition’.
“The discovery of small molecules that bind to and disrupt the function of a specific target is an important step in chemical biology, especially for poorly characterized proteins,” explains Isao Miyazaki, a researcher with Hiroyuki Osada’s team at the RIKEN Advanced Science Institute in Wako. “We call these small molecules ‘bioprobes’.”
Recent work from Miyazaki and Osada demonstrates a highly effective strategy for identifying such bioprobes, which they have used to identify inhibitors of the human pirin protein1. Pirin is conserved across a diverse array of organisms, and has been tentatively linked to cancer malignancy in humans; however, little is known about how this protein might govern tumor progression.
The investigators produced glass slides containing a large array of small molecules, which they then treated with cellular extracts containing pirin protein fused to DsRed, a fluorescent tag. By identifying the spots selectively highlighted by the labeled protein, they were able to zoom in on a candidate molecule with apparently high specificity and binding affinity for pirin, which they named triphenyl compound A (TPh A).
High-resolution structural analysis demonstrated that TPh A inserts itself deeply within the pirin protein, and Miyazaki sees this as proof of the utility of their screening strategy. “There has been a question of whether ligands identified using chemical array approaches typically bind at shallow surfaces,” he says. “Our study confirms that chemical array methods can identify molecules that bind to buried pockets in proteins.”
Accordingly, TPh A appears to act as an effective functional inhibitor. Melanoma cells treated with this compound showed a marked reduction in cell migration, and this effect appears to arise from TPh A-mediated disruption of the interaction between pirin and the cancer-related protein Bcl3. By analyzing cellular gene expression profiles, the researchers subsequently uncovered evidence that pirin and Bcl3 collaborate to switch on the SNAI2 gene, which is known to contribute to tumor progression and metastatic growth.
These findings demonstrate the potential of bioprobe screening as a strategy for uncovering hidden protein functions. Miyazaki and Osada anticipate that TPh A will provide a valuable tool for future investigations of the role of pirin in other cancers and may even prove useful for studying related proteins from other organisms.
The corresponding author for this highlight is based at the Antibiotics Laboratory, RIKEN Advanced Science Institute