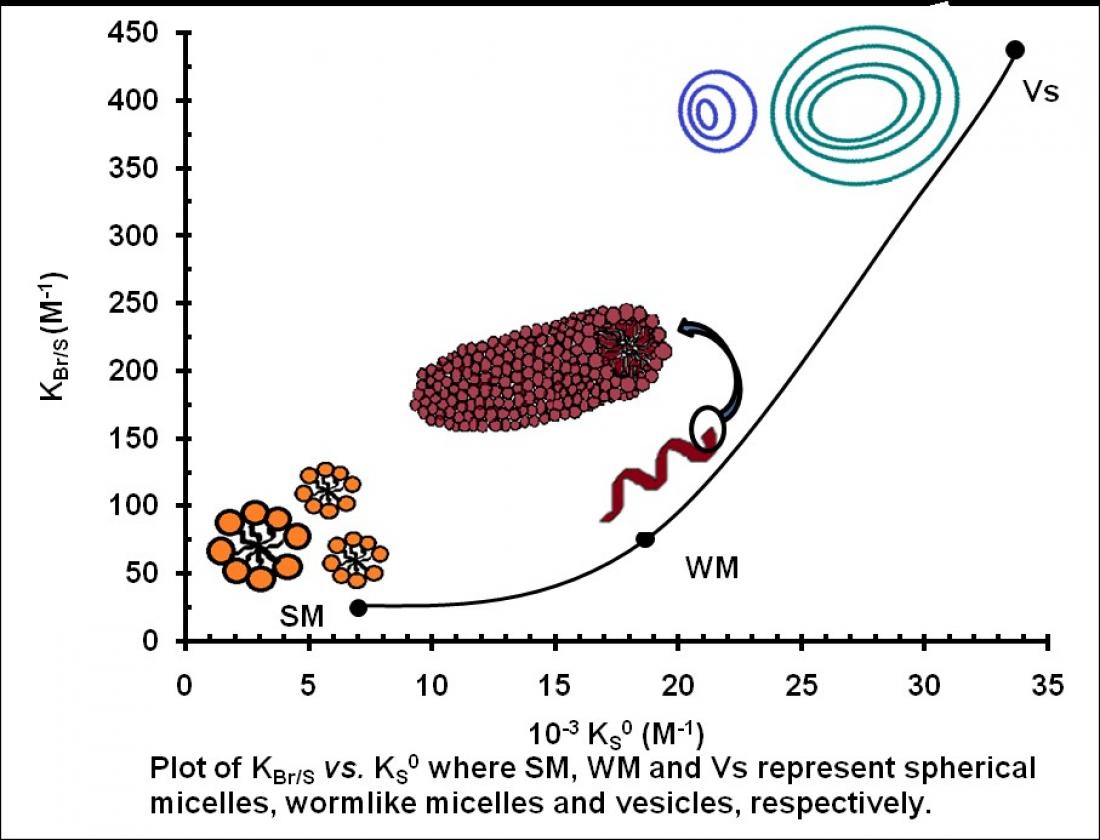
Figure 1. Plot of KBr/S vs. KS0 where SM, WM, and VS represent spherical micelles, wormlike micelles and vesicles, respectively.
The effects of moderately hydrophobic counter ions (X) on cationic micellar growth have been studied extensively because of its various industrial applications. We have developed a chemical method to quantify X affinity to ionic micelles, and X-induced and temperature-induced micellar growth (spherical-to-wormlike micelles-to-vesicles). Such a correlation has a predictive power to predict X-induced and temperature-induced micellar growth. Hence, the finding of this project are expected to benefit the following research communities:
• Data analysis of ionic micellar-catalyzed semi-ionic bimolecular reactions encountered in both applied and fundamental research institutions.
• Detergent and pharmaceutical based industrial processes.
• Molecular separation science
Micelles are dynamic in the sense that their molecular geometry is highly sensitive to the surrounding environment such as surfactant concentration, temperature, pH, ionic strength as well as the presence of additive. The counter ion (X-), especially the substituted benzoate ion and temperature are well known for their contribution in affecting micellar structural changes such as growth from spherical-to-rodlike-to-wormlike-to-vesicle. Thus, the value of micellar binding constant of X-, KX increased in the order: KX (spherical) < KX (rodlike) < KX (wormlike) < KX (vesicle) when measured under the same surfactant (such as DDABr).
Quantitative correlation of counter ions (X-) binding affinity to cationic micelles can be obtained by kinetically determine the relative binding ratios, where X- and Br- are the competing counter ions in the cationic micellar system studied. These ratios are calculated by using an empirical equation, which was derived from on Pseudophase Micellar (PM) model. The reaction systems contain aqueous solution of DDABr micelle and X- were varied according to the organic salt (MνX) used in the system. The determination of relative binding ration involves the determination of a few kinetics parameters using semi-empirical kinetic or spectrophotometric method depending on the conter ions, e.g. X- and S-. In the kinetic method, the probe consists of the piperidinolysis of Phenyl Salicylate (PSa-) in the presence of constant concentration of [DDABr]T and [NaOH], and variable [MνX] concentration.
For further information contact:
Professor Dr. Mohammad Niyaz Khan
Phone: 03-79674163
Fax: 03-79674193
[email protected]
Department of Chemistry
Science Faculty
University of Malaya
50603 Kuala Lumpur
Dr. Nor Saadah Mohd Yusof
Phone: 03-79674052
[email protected]
Department of Chemistry
Science Faculty
University of Malaya
50603 Kuala Lumpur