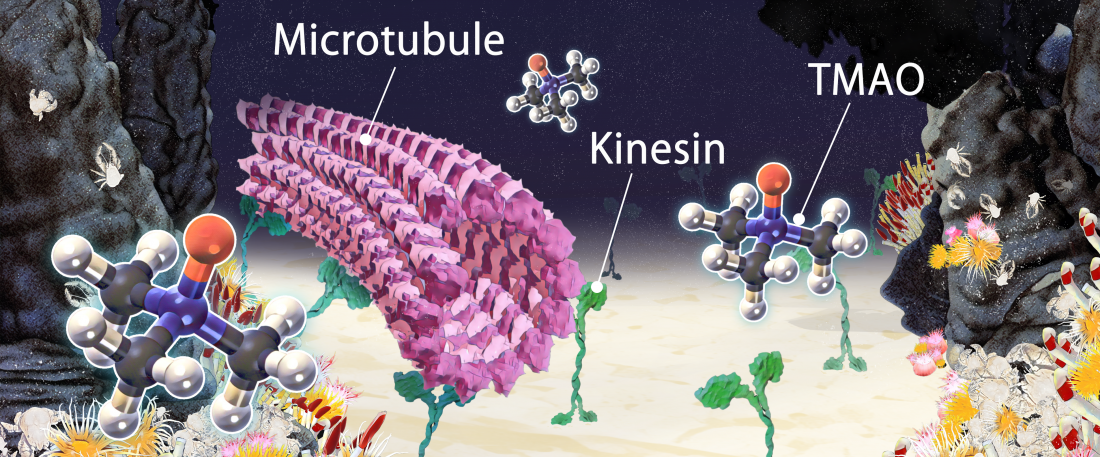
Conceptual illustration showing deep-sea osmolyte TMAO stabilizing the interaction between microtubules and kinesins. (Munmun, T. et. al., Chemical Communications, December 26, 2019)
Biomolecular motors are the smallest natural machines that keep living organisms dynamic. They can generate force and perform work on their own by consuming chemical energy. In recent years, reconstructed biomolecular motors have appeared as promising substitutes of synthetic motors and expected to be key components in biomimetic artificial micro- or nano-devices. However, reconstructed biomolecular motors lose their ability to function due to thermal instability in artificial environments.
Tasrina Munmun, Arif Md. Rashedul Kabir, Kazuki Sada and Akira Kakugo of Hokkaido University and Yukiteru Katsumoto of Fukuoka University were inspired by seeing how proteins remain stable in living organisms such as sharks, teleosts, skates, and crabs that survive in harsh environments like deep sea hydrothermal vents or under thermal perturbations. Although proteins are generally denatured by heat, the proteins in deep-sea animals remain stable and active with heat thanks to TMAO.
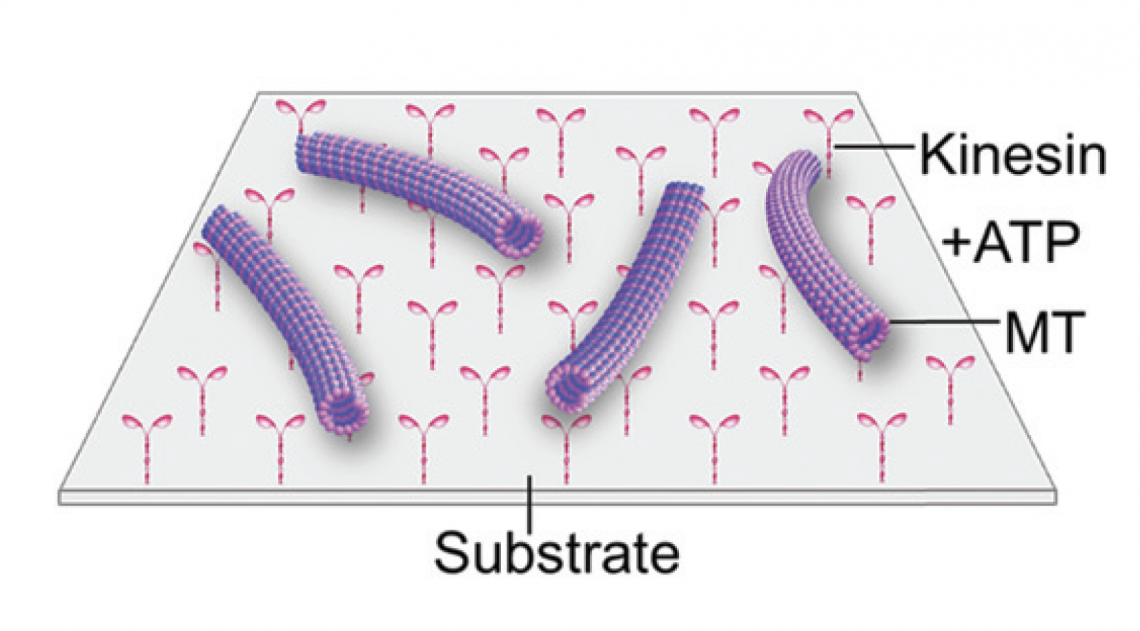
“Based on this fascinating defense mechanism in deep-sea animals, we attempted to control the activity of kinesin, a biomolecular motor associated with microtubule proteins, over a wide temperature range,” said Arif Md. Rashedul Kabir. To investigate the activity of kinesins, the team conducted in vitro motility assays in which kinesin motors propelled the microtubules on a two-dimensional substrate.
In the motility assay, microtubules are propelled by kinesin motors attached to the substrate surface. (Munmun, T. et. al., Chemical Communications, December 26, 2019)
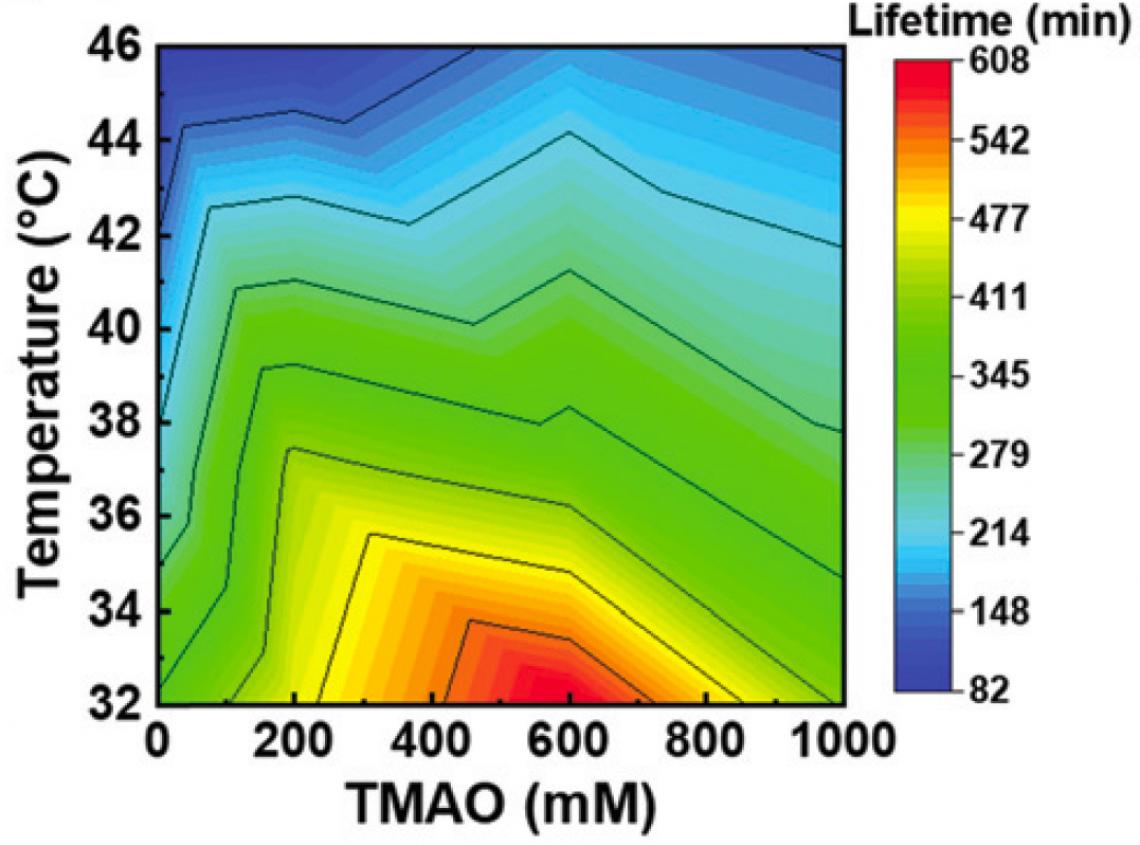
According to the study published in Chemical Communications, they discovered that TMAO suppresses thermal denaturation of kinesins in a concentration dependent manner. Within a temperature range of 22–46 °C, kinesins propelled microtubules for a prolonged time (almost 2.5 times longer) when TMAO was present. This shows the team successfully controlled the dynamics between kinesins and microtubules over a broad temperature range. “This study is the first example showing successful utilization of a deep-sea osmolyte in maintaining biomolecular motors for a prolonged time over a wide temperature range in engineered environments,” Arif Md. Rashedul Kabir commented.
TMAO is effective in prolonging the lifetime of microtubule-kinesin interaction over a wide range of temperatures. (Munmun, T. et. al., Chemical Communications, December 26, 2019)
Arif Md. Rashedul Kabir continued, “The idea of utilizing natural defense mechanisms against heat-induced inactivation of proteins and enzymes will now be encouraged further.”
“Our work will open a new dimension in sustainable applications of reconstructed biomolecules which will benefit various fields including biomimetic engineering, biochemical and biomedical engineering as well as materials science,” Akira Kakugo added.
Funding:
This study was supported by research grants from Hirose International Scholarship Foundation (PK22181027), Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) (18H05423, 18H03673), and Future AI and Robot Technology Research and Development Project from NEDO, Japan.
Contacts:
Associate Professor Akira Kakugo
Graduate School of Chemical Sciences and Engineering
Hokkaido University
Email: kakugo[at]sci.hokudai.ac.jp
Naoki Namba (Media Officer)
Institute for International Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp
From the left: Tasrina Munmun, Arif Md. Rashedul Kabir, Yukiteru Katsumoto and Akira Kakugo of the research team.