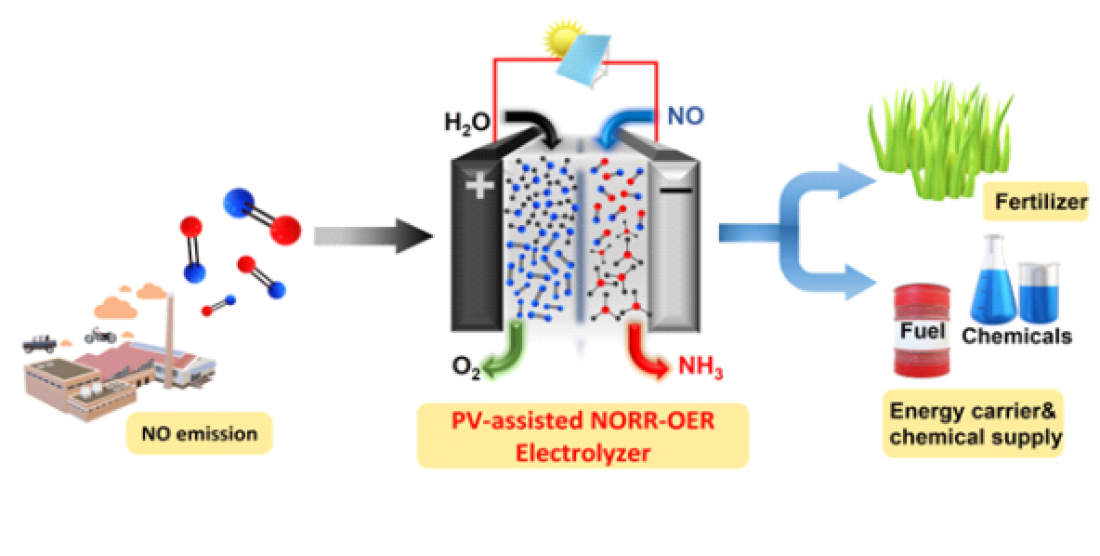
□ DGIST research team, led by Professor Sangaraju Shanmugam, Department of Energy Engineering, developed a catalyst that converts nitric oxide (NO) to ammonia (NH3). This electrochemical technology offers high Faradaic efficiency[1] and low overpotential[2] and produces NH3 in an eco-friendly manner.
□ NH3 is an important chemical raw material in the fertilizer, textile, and pharmaceutical industries, and it is considered a carbon-free hydrogen carrier with a high energy density. Typically, NH3 is produced using the Haber–Bosch process[3]; however, this process is responsible for approximately 1–2% of global CO2 emissions.
□ The electrochemical conversion of NO to NH3, an alternative to the Haber–Bosch process, has received considerable attention. This eco-friendly method consumes the air pollutant NO gas to produce NH3. Therefore, this promising approach can replace conventional methods without affecting the environment or emitting CO2.
□ However, owing to the corrosive nature of NO gas, the morphology of the metal-nanoparticle electrocatalyst degrades during electrosynthesis. Therefore, it is necessary to obtain a catalyst material with high stability that facilitates long-term electrochemical NH3 synthesis.
□ Professor Shanmugam’s research team developed a core-shell nickel nanoparticle coated with nitrogen-doped carbon nanostructured electrocatalysts via a simple co-precipitation method for stable NH3 production. This catalyst is stable and efficient and offers a high Faradaic efficiency of 72.3% at a low overpotential (550 mV) in a 100% NO-saturated electrolyte. Additionally, a solar-to-NH3 efficiency of 1.7% and Faradaic efficiency of more than 50% were achieved using a solar-energy-assisted full-cell PV-NORR electrolyzer.
□ Professor Shanmugam stated, “This research has developed an energy-efficient and eco-friendly technology to synthesize NH3 with zero carbon footprint, and we hope to commercialize this technology to contribute to environmental conservation and sustainability.”
□ This mid-level research project was conducted with support from the Korean National Research Foundation (NRF). The findings of this research were published in Advanced Science, a renowned academic Journal in the field of materials engineering.
Correspondent author's e-mail address : [email protected]
[1] Faradaic efficiency: A measure of the efficiency with which the charge is consumed only for the given electrochemical reaction. A Faradaic efficiency of close to 100% indicates efficient charge consumption for the desired reaction.
[2] Overpotential: It indicates the potential difference between the thermodynamically determined potential of an electrochemical reaction and the potential at which the reaction is observed experimentally.
[3] Haber-Bosch Process: It is a process that can synthesize ammonia in large quantities from the abundant nitrogen in the air, and it triggered the agricultural revolution through the production of synthetic fertilizers using ammonia.