□ The research team led by Professor Hongkyung Lee at DGIST (President Kuk Yang) announced on the 16th (Wednesday) that they have developed a new type of lithium, overcoming the limitations of lithium batteries and potentially replacing existing commercial lithium cathodes through a new manufacturing process.
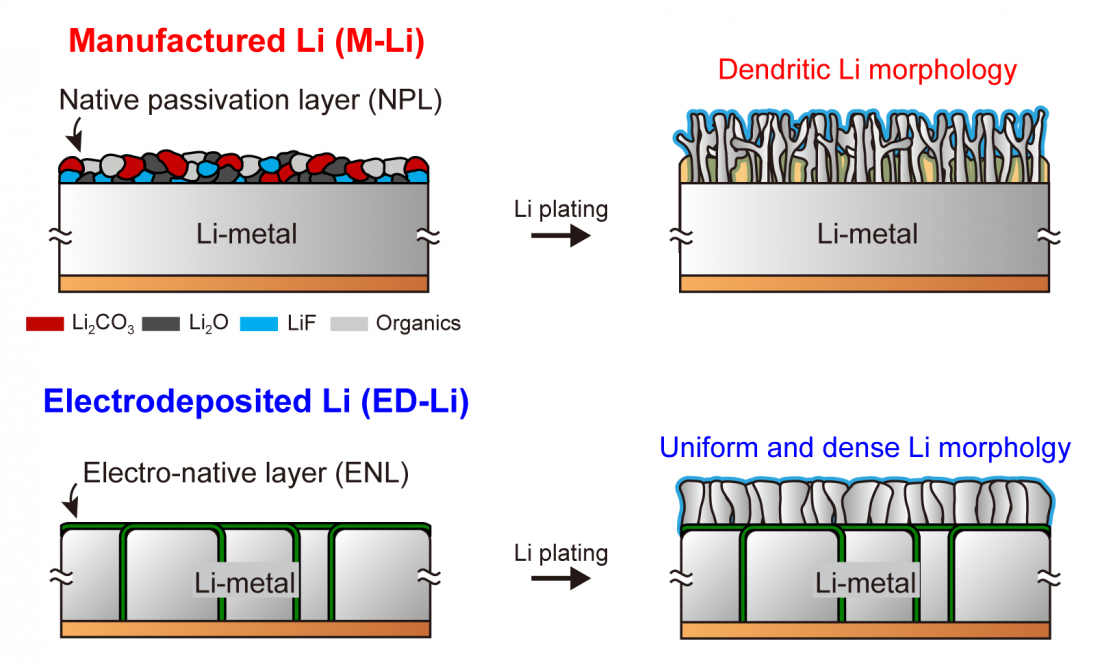
□ Lithium metal, which is widely known as an ideal cathode material for batteries, can be used to create batteries that overcome the energy density limitations of conventional lithium-ion batteries since it has 10 times more theoretical capacity than graphite, which is currently commercialized. Nonetheless, lithium metal is commonly used as lithium foil, which results in the formation of an oxide layer during production and storage. Therefore, lithium dendrite[1] easily forms during charging and discharging as the lithium surface quality is abruptly degraded, which ultimately hinders commercialization.
□ The research team led by Professor Hongkyung Lee thus developed a new manufacturing method for replacing lithium foil. This particular method involves manufacturing a much smoother and more uniform lithium metal cathode by applying electroplating using a pseudo-high-concentration electrolyte. The surface of the lithium created with the pseudo-high-concentration electrolyte contains a high level of fluorine-based substances, thus lowering the reactivity with the electrolyte and allowing it to be manufactured thinly and compactly through simple physical compression. The lithium cathode manufactured accordingly is much smoother than commercial lithium foil, has a more uniform chemical composition on the surface layer, and can be more easily manufactured in large sizes compared to conventional manufacturing processes.
□ The surface layer of the currently available lithium foil contains a large amount of lithium carbonate (Li2CO3) and has uneven chemical compositions. Consequently, the lithium grows irregularly, which causes problems when it comes in contact with the electrolyte. However, the lithium metal developed by Professor Lee’s research team forms an “electro-native layer,” thus having an affinity with the electrolyte and low resistance. As a result, fast and uniform electrochemical reactions are induced, successfully suppressing dendrite growth. Furthermore, lithium is discharged smoothly during discharging, which enables the battery interior to be kept smooth. This enhancement will lead to significant improvements in the performance and stability of lithium batteries when designing commercial batteries.
□ Professor Lee said, “This research has drastically improved the life of lithium batteries by simply improving the initial surface quality of the lithium cathode,” and added “Further research will be conducted to accelerate the commercialization of lithium metal batteries by introducing the surface stabilization technology.”
□ The findings of this research were published online on June 12th in the international energy science journal Energy Storage Materials. A graduate student in the integrated MSc and PhD program, Jiyeon Seo, participated in the research as the corresponding author. This study was supported by the “Excellent Rising Business” program of the National Research Foundation of Korea and the “Nano Convergence and Innovative Product Technology Development Project” of the Ministry of Trade, Industry and Energy.
- Email address of the correspondence : [email protected]
[1] Dendrite growth: Fine line-shaped chunks are formed as metal lithium grows during charging and discharging. Fire or explosion may occur inside the battery as the chunks become larger, thus reducing battery safety.