Angewandte Chemie International Edition: Press release 06/2022
Haze is formed when a cocktail of various gaseous pollutants is oxidized and forms particulate matter diffusing sunlight. This process is mainly mediated by hydroxyl radicals (OH), and researchers have now discovered a new route to their formation. This newly discovered radical-building mechanism could also offer new perspectives for air purification and the energy industry, as the study published in Angewandte Chemie shows.
Haze consists of fine particulate matter containing soot. It is formed when gaseous pollutants, which are from industrial emissions, vehicle exhausts, and other sources, are converted to condensable matter. “This condensation is remarkably accelerated under the action of OH radicals,” says Joseph S. Francisco from the University of Pennsylvania in Philadelphia, USA, who is co-author of the study.
The commonly known sources for OH radicals, such as nitrogen oxide and ozone, only partly account for the vast haze events which keep occurring in haze-afflicted regions such as the megacities of East and South Asia.
In a cooperation, the teams of Hong He at the Chinese Academy of Sciences, Xiao Cheng Zeng at the University of Nebraska-Lincoln, USA, and Francisco have now taken a closer look at the chemical activity of soot particles. Soot originates from diesel engine exhaust fumes or is spread by slash-and-burn practices or forest fires. However, to date, soot particles consisting of uncombusted carbon have been considered more as a sink of hydroxyl radicals, rather than a source.
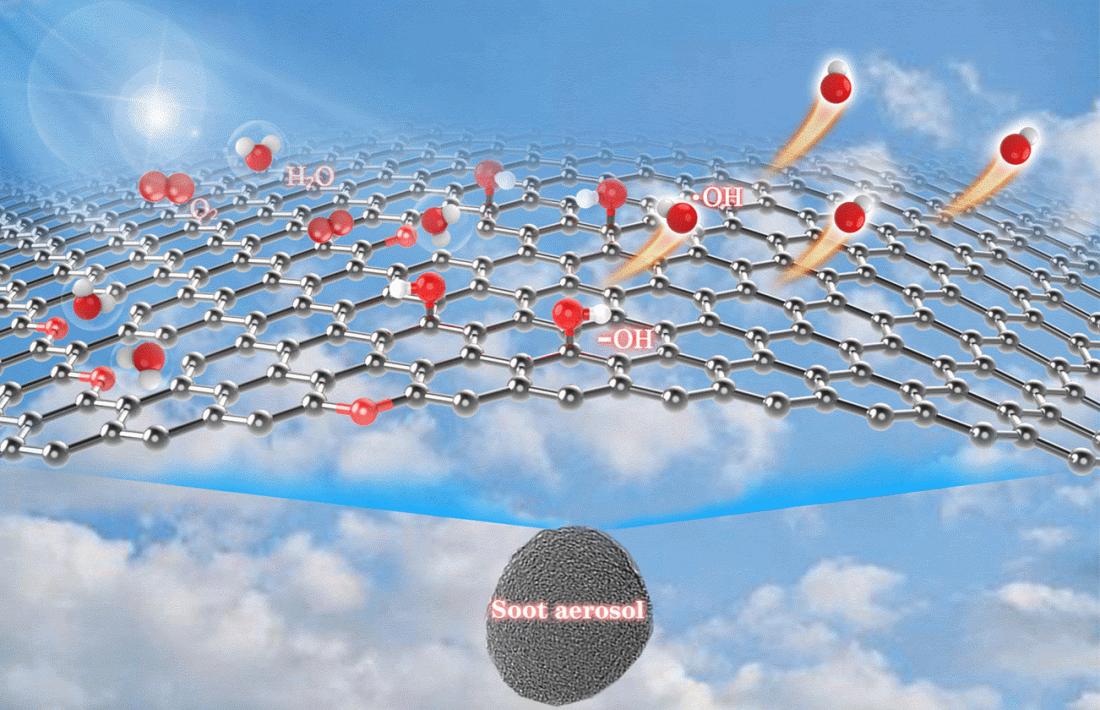
Despite this, Francisco and the team’s new experiments showed that soot particles can produce OH radicals if air and water vapor are blown over the particles while being irradiated with light.
It was expected, though, that hydroxyl species formed in this process would not leave the surface of the soot and would quickly react again. However, energy calculations showed that the hydroxyl exhibited “roaming-like features”, as the authors stated it: they migrated over the surface, ultimately leaving it.
The results of their study led the team to the conclusion that soot particles play an active role in smog formation. But the researchers aren’t stopping there: since it seems that light radiation is sufficient to decompose water molecules into radicals, this material could potentially be used to develop metal-free carbocatalysts. Such soot-based catalysts could either help purify the air from pollutants such as nitrogen oxide and volatile organic compounds (VOCs), or they could be used to generate chemical energy from light energy. This could pave the way for an environmentally friendly form of artificial photosynthesis.
About the Author
Dr. Joseph S. Francisco is President Distinguished Professor at the Department of Chemistry of the University of Pennsylvania. The research in his laboratory focuses on kinetics and photochemistry of novel transient species in the gas phase, in aerosol, and at the ice-quasi liquid layer, to address the questions of how structures correlate to reactivity and photochemical mechanisms.
Dr. Hong He is Professor of the Research Center for Eco-environmental Sciences, Chinese Academy of Sciences, China. His research team focuses on the heterogeneous transformation and elimination of pollutants in environmental catalysis and multi-phase reaction process in atmospheric chemistry. His team develops theories of haze chemistry and the technology for the control of mobile/stationary source of indoor air pollution.
Author: Joseph S. Francisco, University of Pennsylvania (USA), https://www.chem.upenn.edu/profile/joseph-s-francisco
Title: Generation and Release of OH Radicals from the Reaction of H2O with O2 over Soot
Angewandte Chemie International Edition
Permalink to the original article: https://doi.org/10.1002/anie.202201638
Angewandte Chemie is a journal of the Gesellschaft Deutscher Chemiker (German Chemical Society, GDCh) and is published by Wiley-VCH. It is one of the prime chemistry journals in the world.