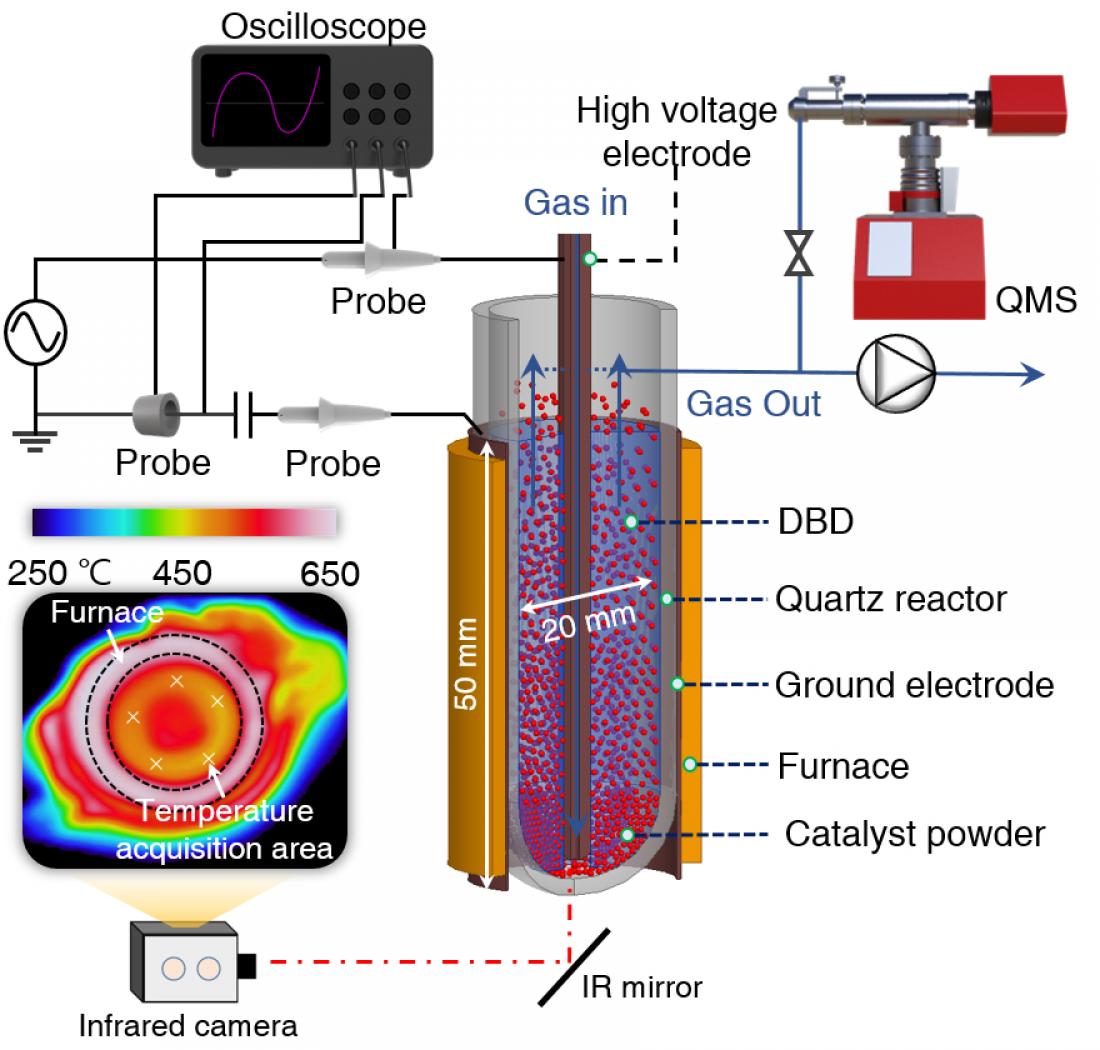
Carbon dioxide recycling—innovative plasma-catalysis concept. Fluidized-bed dielectric barrier discharge reactor was used for CO2 hydrogenation over Pd2Ga/SiO2. Credit: Journal of the American Chemistry
Joint release by Tokyo Institute of Technology, Hokkaido University, and Japan Science and Technology Agency.
Climate change accelerated by excess CO2 emissions has been a major concern over the past few years. To deal with this problem, technologies that can not only reduce and remove excess CO2 emissions but also transform them into value-added chemicals are being developed. One such method is the hydrogenation of CO2 using renewable hydrogen to produce alternative fuels.
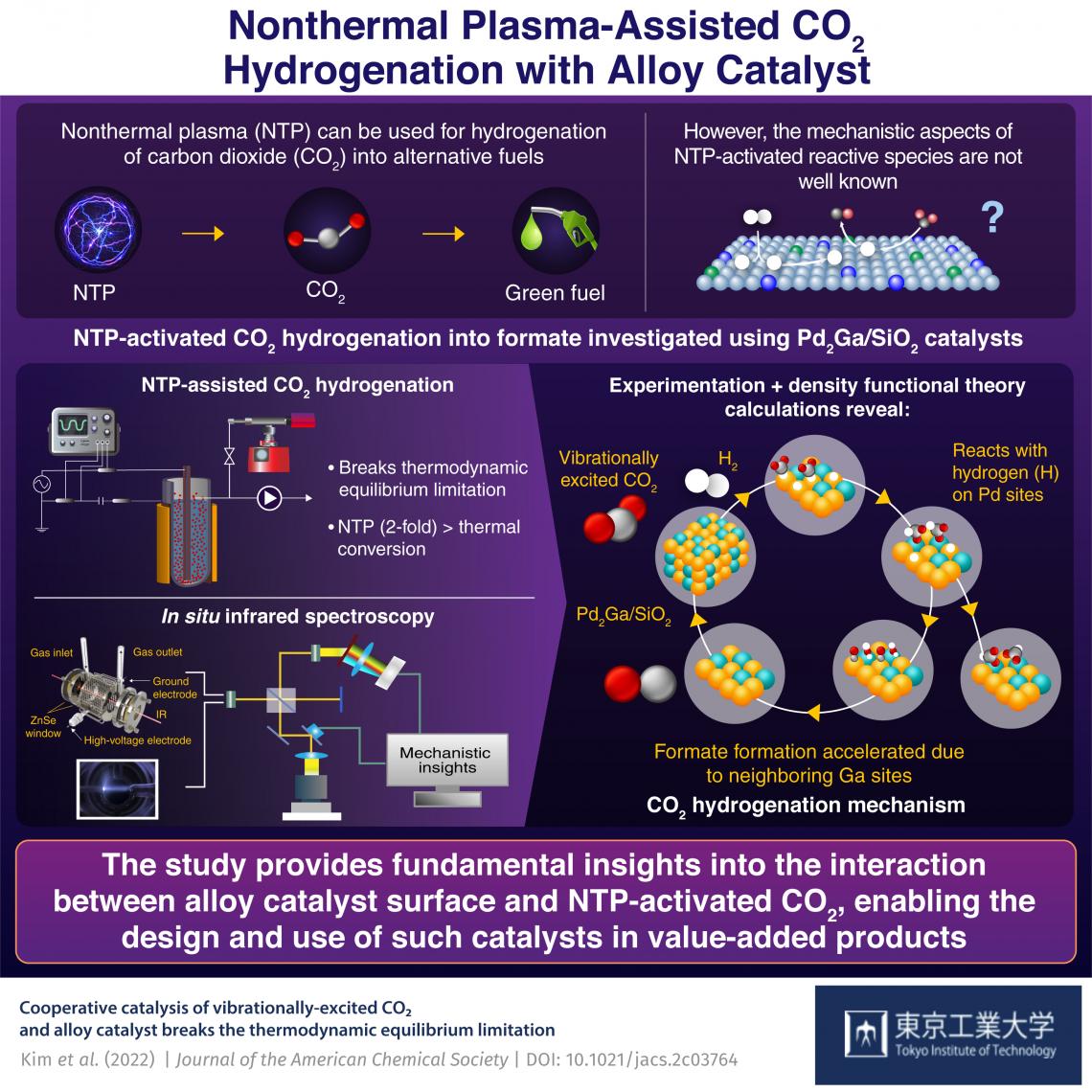
Over the years different strategies have been developed to improve CO2 hydrogenation in the presence of metallic catalysts. The most promising among them is nonthermal plasma (NTP). It promotes hydrogenation of CO2 beyond the thermodynamic limit even at low temperatures without deactivating metallic catalysts, which are vulnerable to higher temperatures. Despite the rising popularity of this technique, the interactions between the NTP-activated species and metallic catalysts are still not well understood.
Fortunately, a team of researchers from Tokyo Institute of Technology (Tokyo Tech), Japan, led by Prof. Tomohiro Nozaki, devised a study to overcome this gap in understanding. In their recent breakthrough, published in the Journal of the American Chemical Society, the researchers revealed the reaction dynamics for NTP-assisted CO2 hydrogenation on the surface of Pd2Ga/SiO2 alloy catalysts that lead to the formation of formate. Prof Nozaki explains “Reaction mechanisms like Eley-Rideal or E-R pathway have been proposed to explain efficient CO2 conversion at lower temperatures and the activation energy for this reaction decreases dramatically. Moreover, NTP produces a copious amount of vibrationally activated CO2 which is the key to enhancing CO2 conversion beyond the thermal equilibrium.”
The team investigated the reactions between NTP activated CO2 and Pd2Ga/SiO2 alloy catalysts in a fluidized-bed dielectric barrier discharge reactor (Figure 1 and Videos) and compared them to conventional thermal catalysis. The results revealed that the CO2 conversion into formate was more than two-fold in the case of NTP-assisted hydrogenation when compared to thermal conversion. To further establish the mechanics of the mentioned conversion, the scientists adopted in situ spectroscopic analysis and density functional theory (DFT) calculations.
The results revealed that the NTP activation gave rise to vibrationally excited CO2 molecules which directly react with hydrogen atoms adsorbed by the Pd sites on the catalyst via the E-R pathway. One of the O atoms from the reacted species then got adsorbed at the neighboring Ga site resulting in the formation of monodentate-formate or m-HCOO. The DFT calculations also deduced a decomposition pathway for the same m-HCOO species.
This experimental-theoretical study showed that NTP can promote CO2 hydrogenation to limits those conventional thermal methods can hardly reach. It also provided mechanistic insights into NTP activated CO2 and catalyst interaction, which can be utilized to develop better catalysts and improve the hydrogenation process. “With our research, we wanted to accelerate the waste to wealth initiative. Capturing CO2 and using it as feedstock for synthesis of fuels and valuable chemicals will not only help us deal with climate problem but also slow down fossil fuel depletion to some extent,” concludes Prof. Nozaki.
Credit: Professor Tomohiro Nozaki, Tokyo Institute of Technology
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in the industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
https://www.titech.ac.jp/english/
About Hokkaido University
Founded in 1876 as Sapporo Agricultural College, Hokkaido University is one of the oldest, largest, and most prestigious universities in Japan. The university attracts prospective students all around the globe with the diverse degree programs offered and the all year round scenic beauty. The campuses are located in the cities of Sapporo and Hakodate of Hokkaido and 21 facilities are spread throughout Hokkaido and mainland Japan, contributing towards the resolution of global issues.
https://www.global.hokudai.ac.jp/
About Japan Science and Technology Agency (JST)
JST is an organization that leads Japan’s science and technology (S&T) development as an innovation navigator. We aim to contribute to the lives of people and the achievement of a sustainable society by promoting S&T for the purpose of opening up opportunities in innovation. Since its foundation, JST’s many outstanding achievements accomplished in collaboration with the government, universities, the industrial sector and public have been earned global recognition.
https://www.jst.go.jp/EN/