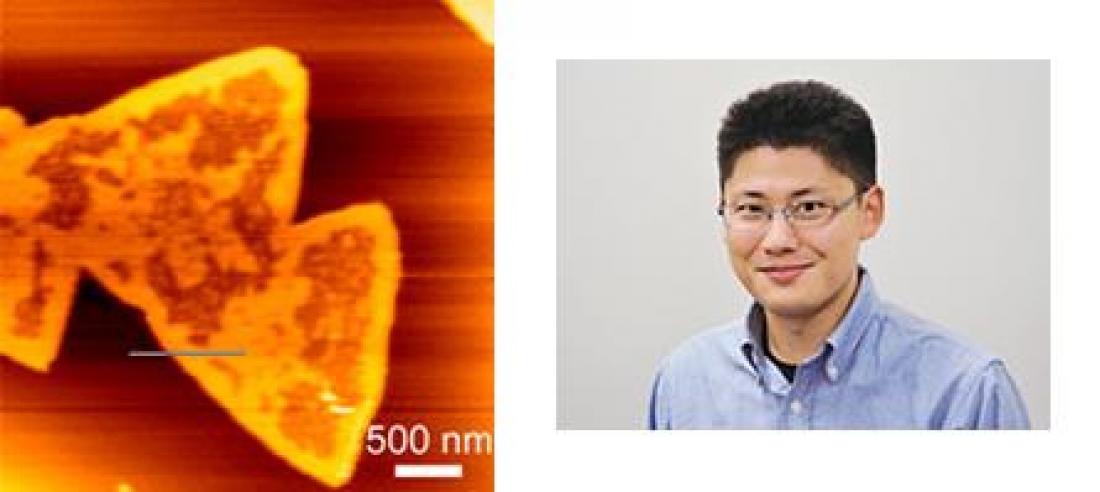
Dr Tero and Figure 1: AFM topograph of the triangular domains GM1+SM+Chol-SLB on a mica surface.
Ganglioside GM1 (GM1), a glycosphingolipid responsible for neuronal plasticity and repair mechanism, works as a seed for the polymerization of amyloid β (Aβ) peptide, which is regarded as one of fundamental processes of Alzheimer's disease.
However, lateral organization of GM1 molecules in cell membranes, such as molecular clustering and domain formation, is still unclear.
Here, Ryugo Tero at the Electronics-Inspired Interdisciplinary Research Institute (EIIRIS) in collaboration with Yanli Mao and other colleagues at Institute for Molecular Science found unique phase separation forming triangular domains to occur in a supported lipid bilayer (SLB) consisting of GM1, sphingomyelin (SM) and cholesterol (Chol) at 20:40:40 molar ratio (Fig.1).
The GM1+SM+Chol-SLB was prepared on mica and SiO2/Si(100) surfaces by vesicle fusion method, where the substrate was immersed at the GM1+SM+Chol-vesicle (=liposome) suspension at 70ºC, and the adsorbed vesicles on the substrate surface spontaneously transformed to SLB. The SLB was again incubated at 37ºC for 24 h and observed by using atomic force microscope (AFM).
Triangular domains appeared only on mica surface, not on SiO2/Si(100), and all triangles were in the same direction, therefore the phase separation was induced by the SLB-mica interaction. Cholera toxin B-subunit (CTXB) assay to GM1 revealed GM1 to localize at the region out of the triangular domains. The researchers also found that the GM1+SM+Chol-SLB on mica was highly active for Aβ-polymerization.
These results open the possibility that solid surface functions may be used to control the biological activity of artificial bilayer membranes in vitro as well as their structures.
For references, please click on the link below.
*Ryugo Tero is now at the Electronics-Inspired Interdisciplinary Research Institute (EIIRIS), Toyohashi University of Technology.