Press Release
Source: Kanagawa University, Japan, Organization of Frontier Science and Innovation
For immediate release: 25 June 2013
A better route to hydrocarbazoles
(Kanazawa, Japan, 25 June 2013) A new chemical process developed by researchers at Kanazawa University provides an efficient way of synthesizing diverse organic compounds. This research is also described in the inaugural June 2013 issue of the Kanazawa University Research Bulletin: http://www.kanazawa-u.ac.jp/research_bulletin/index.html
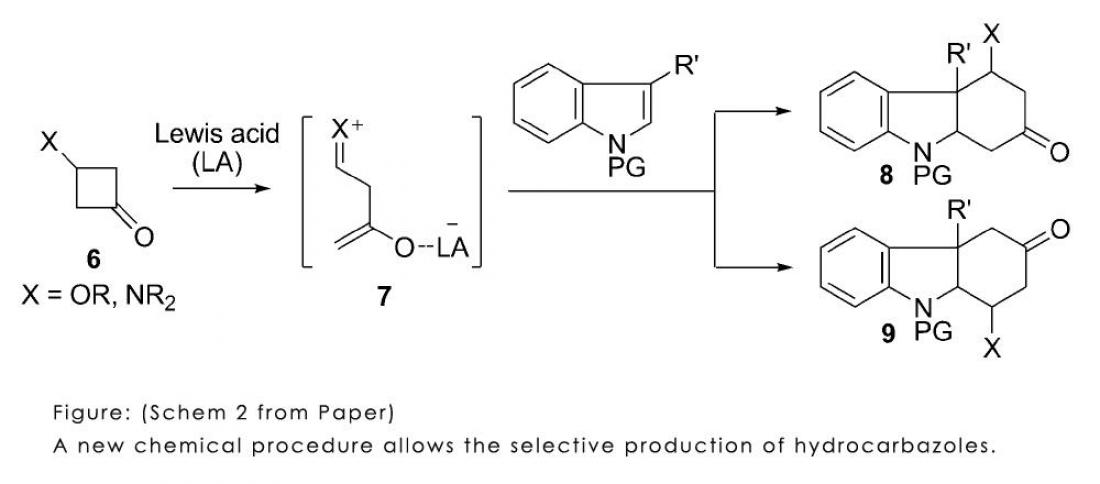
Jun-ichi Matsuo and co-workers in the School of Pharmaceutical Sciences aimed to produce a wide variety of aromatic compounds called hydrocarbazoles, which are found in many natural substances such as strychnine. Hydrocarbazoles contain a benzene ring linked by a six-membered carbon ring and a five-membered carbon ring containing nitrogen.
Various methods exist for synthesizing hydrocarbazoles by reacting electron-accepting compounds, called enophiles, with indoles – common aromatic structures that are found in flower scents. Because this reaction involves the addition of a cyclic carbon ring, it is called cycloaddition.
Previous cycloaddition methods have been restricted to certain combinations of indoles and enophiles. Moreover, it has been difficult to control exactly where on the molecules the bonds will be formed, and thus what exact chemical structure will come out.
The new process developed by Matsuo and co-workers involves a reaction of cyclobutanones with so-called Lewis acids – molecules that can accept two electrons - to produce an intermediary molecule that has positively- and negatively-charged parts, but zero charge overall. This intermediary can react with many different indoles to produce hydrocarbazoles.
The choice of indole can lead to different types and structures of hydrocarbazoles. So far, the researchers have synthesized structural parts of strictamine molecules, and demonstrated total synthesis of aspidospermidine. Such an efficient, flexible route to these useful compounds could greatly benefit pharmaceuticals and other industries.
Publication and Affiliation
Mizuki Kawano, Takaaki Kiuchi, Shoko Negishi, Hiroyuki Tanaka, Takaya Hoshikawa, Jun-ichi Matsuo*, & Hiroyuki Ishibashi. Regioselective Inter- and Intramolecular Formal [4+2] Cycloaddition of Cyclobutanones with Indoles and Total Synthesis of (±)-Aspidospermidine. Angewante Chemistry Int. Ed. 52 (2013).
School of Pharmaceutical Sciences Institute of Medical, Pharmaceutical, and Health Sciences Kanazawa University, Kanazawa, Japan
*corresponding author, e-mail address: [email protected]
Further information
Organization of Frontier Science and Innovation
Kanazawa University
Kakuma, Kanazawa, Ishikawa 920-1192, Japan
E-mail: [email protected]
Website: http://www.o-fsi.kanazawa-u.ac.jp/en/about/
About Kanazawa University
As the leading comprehensive university on the Sea of Japan coast, Kanazawa University has contributed greatly to higher education and academic research in Japan since it was founded in 1949. The University has three colleges and 16 schools offering courses in subjects that include medicine, computer engineering, and humanities.
The University is located on the coast of the Sea of Japan in Kanazawa—a city rich in history and culture. The city of Kanazawa has cultivated a highly respected intellectual profile since the time of the Kaga fiefdom (1598–1867). Kanazawa University is divided into two main campuses: Kakuma and Takaramachi for its approximately 12,200 students including 500 from overseas.
Kanazawa University website: http://www.kanazawa-u.ac.jp/e/index.html