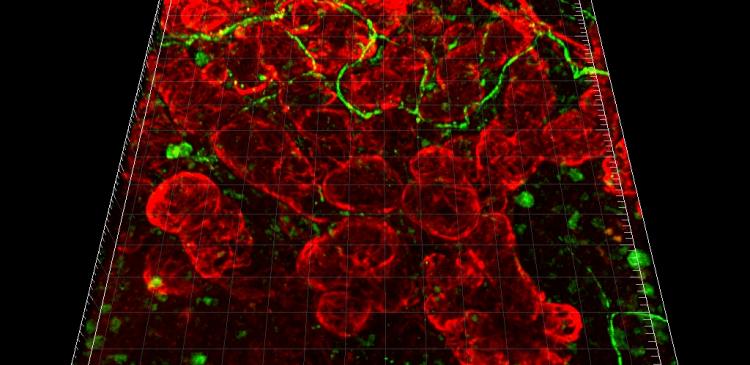
Bioengineered Salivary Gland (green, GFP-epithelial cells and TRITC-gelatin-injected)
Current advances in regenerative therapies have been influenced by the study of embryonic development, stem cell biology, and tissue engineering technologies. The ultimate goal of regenerative therapy is to develop fully functional bioengineered tissues that can replace lost or damaged organs following disease, injury or aging. A research group led by Professor Takashi Tsuji (Professor in the Research Institute for Science and Technology, Tokyo University of Science, and Director of Organ Technologies Inc.) has provided a proof-of-concept for bioengineered mature organ replacement as a regenerative therapy.
Dr. Tsuji’s research group (M. Ogawa et al.) reports the fully functional regeneration of a salivary gland that reproduces the morphogenesis induced by reciprocal epithelial and mesenchymal interactions through the orthotopic transplantation of a bioengineered salivary gland germ as a regenerative organ replacement therapy. The bioengineered germ developed into a mature gland through acinar formations with the myoepithelium and innervation. The bioengineered submandibular gland produced saliva in response to the administration of pilocarpine and gustatory stimulation by citrate, protected against oral bacterial infection and restored normal swallowing in a salivary gland defect mouse model. Thus, this study provides a proof-of-concept for bioengineered salivary gland regeneration as a potential treatment for xerostomia.
This research was performed in collaboration with Professor Tetsuhiko Tachikawa (Department of Oral Pathology, Showa University School of Dentistry, Shinagawa-ku, Tokyo, JAPAN).
BACKGROUND TO THE RESEARCH
1. The role of salivary gland.
Salivary glands play essential roles in normal upper gastrointestinal tract function and oral health, including the digestion of starch by salivary amylase, swallowing and the maintenance of tooth hard tissues through the production of saliva. There are three major salivary glands – the parotid, submandibular and sublingual glands – as well as minor salivary glands. Salivary glands are composedof duct, acinar, and myoepithelial cells (Figure 2 - see attached file).
2. The disease of salivary gland.
Salivary gland impairment, which results from various physiological conditions including radiation therapy for head and neck cancer, Sjögren's syndrome and aging, leads to acinar cell damage and salivary gland hypofunction, including xerostomia (dry mouth syndrome). Xerostomia causes various clinical problems in oral health, including dental decay, bacterial infection, mastication dysfunction, swallowing dysfunction, dysgeusia and a general reduction in quality of life. Xerostomia patients account for one quarter of the total population, and xerostomia is recognised as an important issue in oral and general health. Current therapies for xerostomia involve the administration of artificial saliva substitutes and sialogogues, which are drugs or substances that increase the flow rate of saliva. However, these saliva substitutes and drugs cannot restore salivary gland function. Therefore, a novel therapeutic treatment for the restoration of salivary gland function is needed.
3. Regeneration of salivary gland.
One concept that may be applicable for restoring salivary glands is regenerative therapy, which involves tissue stem-cell transplantation to restore damaged tissues and organs in a variety of diseases. Furthermore, organ replacement regenerative therapy, which can replace lost or damaged organs with a fully functional bioengineered organ, is also expected to provide a novel therapeutic strategy for organ transplantation. Our recent studies have provided proof-of-concept that fully functional regeneration of ectodermal organs, such as teeth and hair follicles, can be achieved by the transplantation of bioengineered organ germs that were reconstituted by our organ germ method (Nat. Methods 4, 227-30, 2007) for organ replacement regenerative therapy (PNAS 106, 13475-13480, 2009, Nat. Commun., 3, 784, 2012) (Figure 3 - see attached file).
OUTLINE OF THE RESEARCH OUTCOME
In this study, the regeneration of a bioengineered salivary gland, which reconstituted with the epithelium and mesenchyme interaction by organ germ method, is indicated through the orthotopic transplantation of a bioengineered salivary gland germ. (Figure 4 - see attached file).
1. Generation of a bioengineered salivary gland germ.
We demonstrated that each bioengineered salivary gland germ, including the submandibular and sublingual glands, had the potential to regenerate into mature glands using our previously developed organ germ method. After three days in organ culture, all of the bioengineered salivary gland germs underwent branching morphogenesis, followed by stalk elongation and cleft formation (Figure 5 - see attached file).
2. Engraftment of a bioengineered salivary gland
To restore proper salivary gland function, a bioengineered salivary gland germ can be engrafted and developed through integration of the host salivary duct and the bioengineered salivary duct. The bioengineered salivary gland germ was transplanted using our previously developed inter-epithelial tissue-connecting plastic method, with a guide for duct direction inserted into the bioengineered germ. Bioengineered salivary glands developed in vivo with the correct connection to the recipient parotid gland duct, as confirmed by the transport of Evans Blue from the host parotid duct to the transplanted bioengineered salivary gland without leaking (Figure 6 - see attached file).
3. Histological analysis of a bioengineered salivary gland
Saliva, which is produced from serous- and mucous-type acinar cells, plays essential roles in oral function. Histological analysis using haematoxylin and eosin (HE) staining and periodic acid and Schiff (PAS) staining revealed a distinctive acinar structure, including serous acinar cells, in the bioengineered submandibular gland and mucous acinar cells in the bioengineered sublingual gland (Figure 7a). E-cadherin and calponin proteins were detected in the acinar and duct cells as well as in myoepithelial cells, which enveloped acinar cells in these bioengineered glands (Figure 7b). Innervations were also detected in the interstitial tissue among acinar cells, and neurofilament H (NF-H)-expressing nerve fibres connected to calponin-positive myoepithelial cells (Figure 7c). Our research demonstrates that engrafted bioengineered salivary gland germ cells successfully formed correct tissue structures and could secrete saliva in response to neural stimulation.
4. Functional analysis of bioengineered saliva secretion
Saliva secretion is an essential function of salivary glands that is critical for maintaining oral and general homeostasis and should be restored by salivary gland regeneration. The central nervous system controls saliva secretion (Figure 8a). We analysed biological salivary secretion using gustatory stimulation with citrate. The engrafted bioengineered salivary glands secreted significant quantities of saliva in response to citrate stimulation via afferent and efferent neural networks. These results indicate that the bioengineered salivary gland secreted saliva via the proper nerve innervations and neurotransmission under the control of the central nervous system (Figure 8b).
5. Amelioration of dry mouth symptoms by saliva secretion
Saliva secretion is essential to the maintenance of oral health, and xerostomia causes various health problems, including dental caries, periodontal disease, bacterial infection and swallowing dysfunction. To semiquantitatively analyse saliva secretion, we performed fluorescein diffusion analysis. Briefly, fluorescent sodium test paper, which contains a fluorescent dye that dissolves in saliva drippings, was placed on the tongue. Dye diffusion was not observed during the observation period in the salivary gland defect mice (Figure 9a and b). Gradual diffusion and disappearance of the fluorescein dye in the oral cavity was observed in mice transplanted with the bioengineered submandibular gland (Figure 9a and b). The number of oral bacteria was drastically increased in salivary gland defect mice compared to normal mice (Figure 9c). The number of bacterial colonies that formed in the mice engrafted with the bioengineered submandibular gland germ was significantly reduced compared to the number in salivary gland defect mice (Figure 9c). These results indicate that the bioengineered submandibular gland has a cleansing function that prevents dryness and inhibits bacterial growth by secreting saliva into the oral cavity.
6. Functional recovery of swallowing and survival
Among salivary gland functions, swallowing is critical for nutrition and reducing the risk of aspiration, which can cause chronic lung disease and affect survival and quality of life, including health and aging. After extracting the salivary glands from model mice, their body weight abnormally decreased (Figure 10a), and all mice died within five days of the surgery (Figure 10b), even though they were supplied with sufficient food and water. The feeding of high-viscosity water prevented the decrease in body weight and survival rate of salivary gland defect mice. Thus, the symptoms observed after the extraction of the salivary glands can likely be attributed to swallowing dysfunction. Body weight initially decreased within two days in mice transplanted with bioengineered submandibular gland germs; however, it recovered and increased four days after transplantation, which is the approximate time needed for the engrafted germ to develop into acini in vivo (Figure 10a). All of the transplanted mice survived, and the survival rate dramatically improved (Figure 10b). These results indicate that saliva secretion from bioengineered salivary glands restored swallowing function associated with the maintenance of oral and general health.
CONCLUSION
Here, we demonstrate the regeneration of fully functional salivary glands through the orthotopic transplantation of a bioengineered salivary gland germ in adult mice. The bioengineered submandibular gland, which was transplanted using an inter-epithelial tissue-connecting plastic method, produced saliva in response to the administration of gustatory stimulation by citrate, protected against oral bacterial infection and restored swallowing in a mouse model of a salivary gland defect. Thus, this study provides a proof-of-concept for bioengineered salivary gland regeneration as a potential treatment of xerostomia. Further studies on the identification of stem cells as a source for the reconstitution of bioengineered salivary gland germs are warranted.