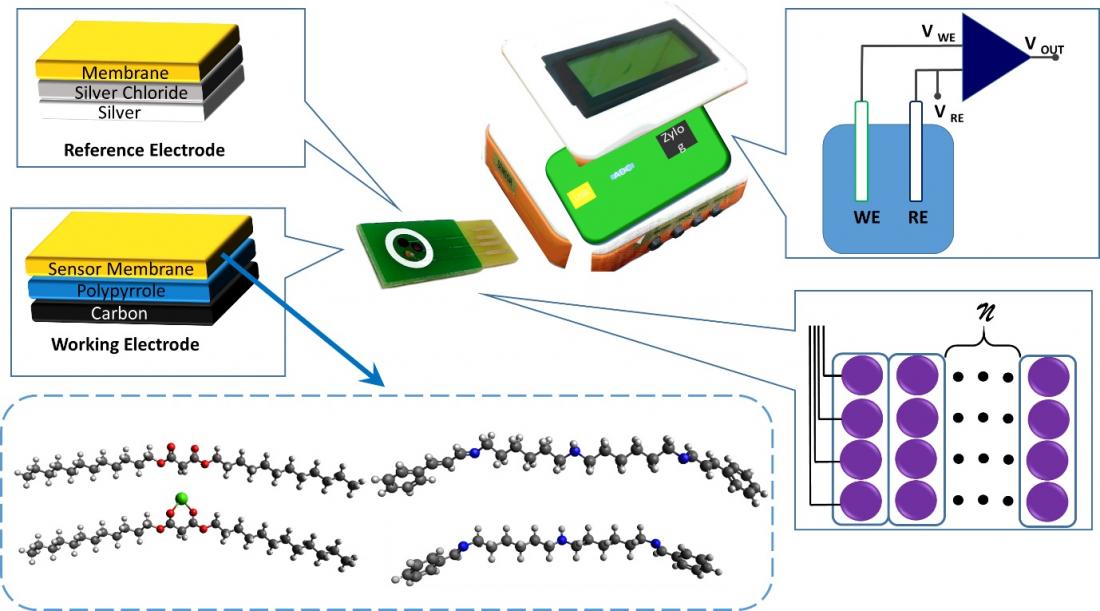
Figure 1 - In-house developed potentiometry reader for multi-ions detection
Multiple sensing cells built from non-selective sensing membrane can be adopted to produce sensor signals that can be fed as variables into a multivariate analytical algorithms. In one of the most popular chemometric approaches, the data is reduced to key principal components which plot the data scores on its principal axes or the magnitude of its vector (loadings). This approach is particularly suitable in the identification of branded formulations (e.g. perfumes, petrol), drinks (e.g. wines and juices) and fertilizer compositions. Adulteration or contamination present in the standard mixtures can be rapidly detected using this approach. Likewise, facile diagnosis on health conditions can be made based on analysis of body fluids, in which the samples obtained from the unhealthy subjects could easily be discriminated from the healthy subjects.
The key elements of this technology are based on printed carbon electrode which promises low manufacturing costs, high quality inert material and excellent substrate for highly reproducible doped conductive polymer electrochemical transducer (e.g. polypyrrole). Researchers at the University of Malaya, Malaysia, leverage widely adopted plasticized PVC membrane and self-plasticized acrylate which emphasize on the manipulation of durability, polarity, and softness of the membrane by exploiting monomers such as n-butyl acrylate and tetrahydrofurfuryl acrylate. Both plasticized and acrylate membrane are doped with tetraphenyl borate function as anion exclusion agents.
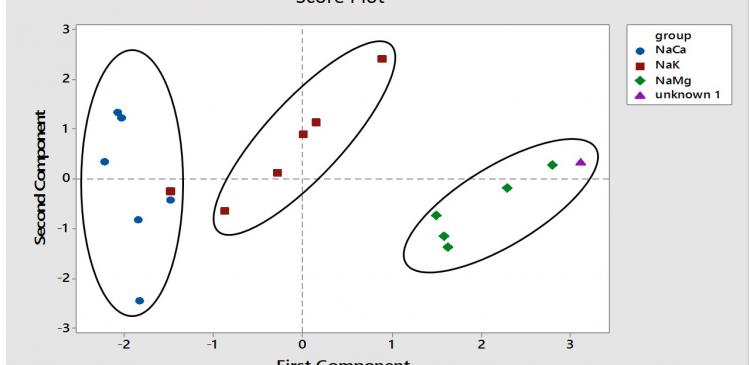
In the early phase of this study, the intrinsic response of these membranes were examined in the absence of chemical agents in the initial stage (prior to addition of chemical agent and ionophore). In the following steps, a suitable amount of lipophilic borate with equimolar of ionophore are optimized. The optimized potentiometry cells are then repeatedly tested in golden standard calibration solutions (contain one ion only). These plots revealed chemical response - albeit low in preferential discrimination. Linking of unique signal patterns to standard mixture composition or branded formulation is the next crucial step. Numerous repeated cycles and replicates are executed in order to establish a reproducible bar charts. In the example below, steps heights of these mixtures (the steps between two bars) are taken as variables for principal component analysis for three standard fertilizer’s formulation. In the final stage of this study, an unknown cation in the mixtures was identified using this method and more studies need to be carried out in order to confirm the source of the unidentified cations.
For further information contact:
Professor Yatimah Alias
Dr. Woi Pei Meng
Chemistry Department
Faculty of Science
University of Malaya
Telephone: +603 7967 7921/4271
Email: [email protected]
Email: [email protected]