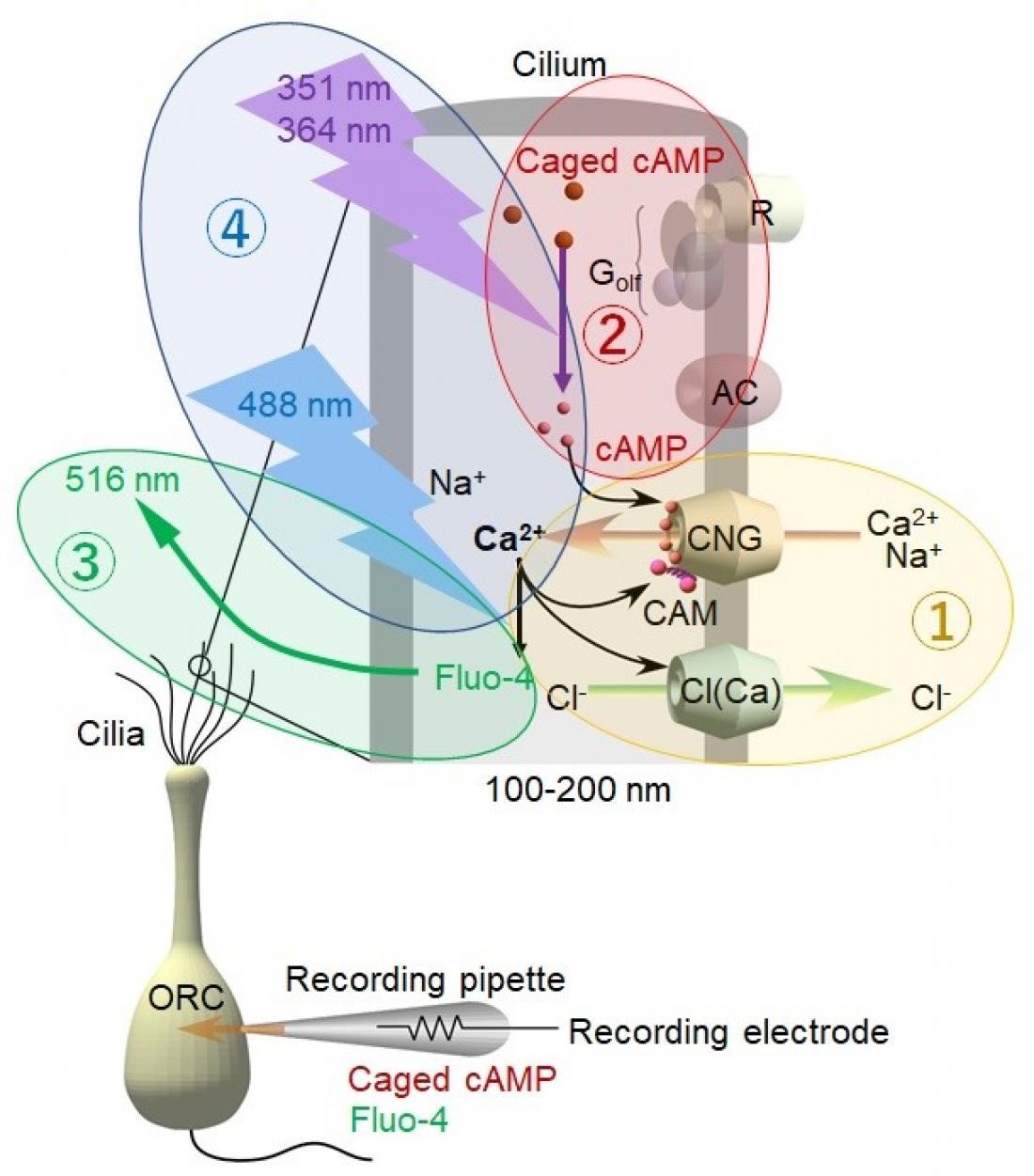
Fig. 1
Experimental scheme
Researchers from Osaka University observe how the segregation of Ca2+ signaling controls the sense of smell
Osaka, Japan- The secrets to the sense of smell are so puzzling that we struggle to ‘sniff out’ the missing pieces. Now, a group of researchers from Osaka University has shown how the segregated function of a messenger ion (Ca2+) can help to improve our sense of smell.
In a study published this month in the Journal of General Physiology, researchers revealed that the amplification and reduction of the sensory signal are both regulated by Ca2+, and the processes are clearly segregated within a tiny structure of a sensory cell.
The initial event that induces the sense of smell is the binding of odorant molecules to the hair-like structures, cilia, attached to the olfactory receptor neurons in the nose. This binding triggers the flow of ions causing an electrical excitation in the cilia, controlled by ion channels that promote the influx of extracellular positive ions into the semifluid compartment of the cilia.. As a result, the Ca2+ signal simultaneously performs the opposing effects of excitation and adaptation to regulate the activity of ion channels on the cilia. A series of events leading to the initiation of a biochemical cascade promotes the increase of Ca2+ inside the cilia: the current increases, impulses are generated, and information is sent to the brain to identify the scent. In the adaptation process, negative feedback signaling occurs to prevent saturation of ion channel activities and to adjust sensitivity to stimulus intensities.
“How the same ion controls both the increase and decrease in local current has been a mystery,” explains lead author Hiroko Takeuchi. “In this research, we aimed to determine whether the process of signal amplification and reduction is indeed segregated only inside the cilia.” The phenomenon remains a mystery because of the technical difficulty in observing the change in Ca2+ dynamics inside the thin structure of the cilia.
The researchers used a novel system to record channel activity and Ca2+ signaling simultaneously. The system applied four techniques: (1) visualization of Ca2+ dynamics in real-time using a Ca2+-sensitive dye, (2) measurement of current across the ciliary membrane (via ion channels), (3) UV activation of ion channels (through a photo-released substance) and (4) employment of confined laser beams for both the stimulation and imaging of the local cilium.
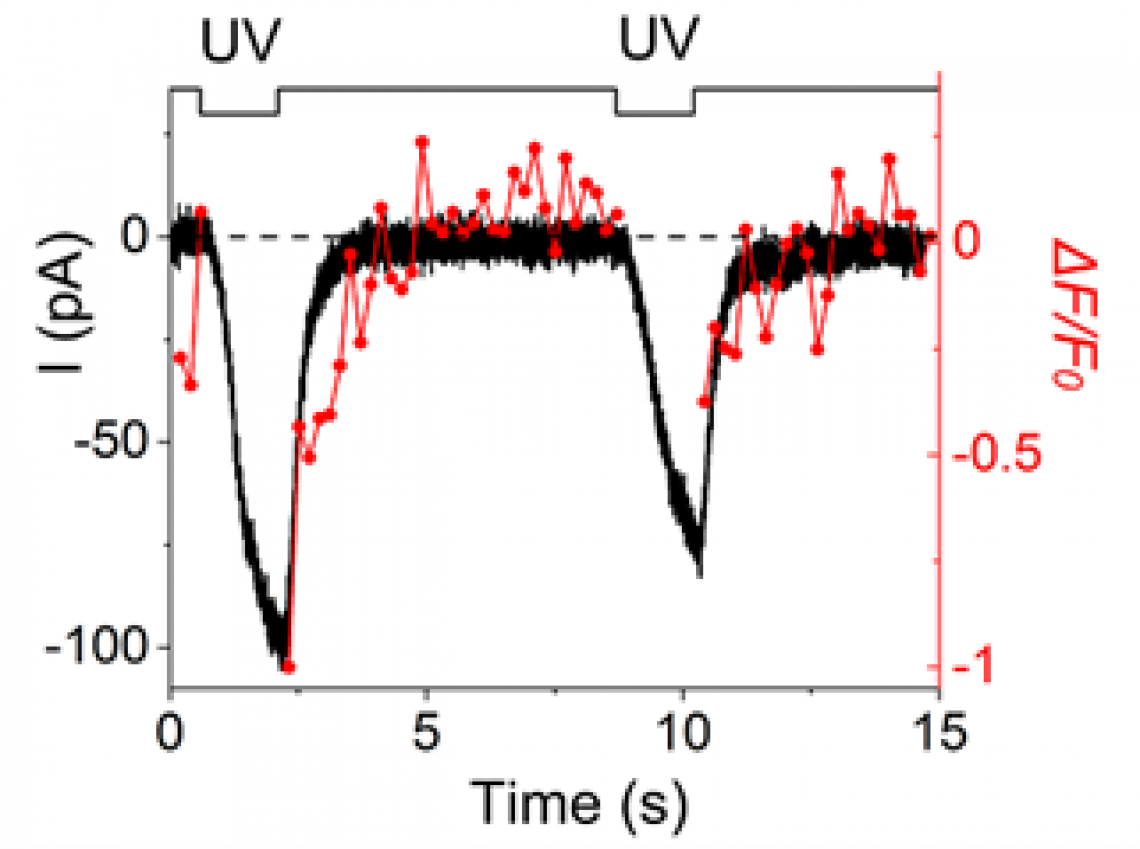
UV excitation of the local cilium immediately generated the inward current and Ca2+ signal; however, after the termination of the stimulus, the current decreased together with the reduction of Ca2+ signal. Surprisingly, however, the adaptation persisted for longer periods even after the Ca2+ signal disappeared. It seems likely that Ca2+ is kept bound exclusively to proteins that regulate the adaptation. “Our results were promising; these opposing effects of Ca2+ occurred separately by molecular kinetics in a tiny space of the native cilium,” says senior author Takashi Kurahashi.
The successful capture of ion channel activity and Ca2+ dynamics within the cilia is a key step toward understanding the human sense of smell. By elucidating the intricacies of Ca2+ signaling, the human sense of smell could be enhanced through the development of olfactory sensors or adjustments to the chemical environment of the nose.
###
The article, “Segregation of Ca2+ signaling in olfactory signal transduction,” was published in the Journal of General Physiology at DOI: https://doi.org/10.1085/jgp.202213165
Fig. 2
Direct comparison of time courses for membrane current and Ca2+ signal in the same location of the cilium.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en