The relationship between the deformation of microtubules and their biological functions may explain incidents that involve mechanical stress on cells. (Syeda Rubaiya Nasrin, Tanjina Afrin, et al., ACS Applied Bio Materials. March 5, 2020)
Joint press release by Hokkaido University and the National Institute of Information and Communications Technology (NICT).
Microtubules are tube-like proteins that act like train tracks for other cellular proteins to move along as they transport molecular cargo. In certain diseases, they become deformed, affecting this transport process. But scientists hadn’t developed an experimental set-up that allows them to properly study this.
In the study published in ACS Applied Bio Materials, biophysicist Akira Kakugo of Hokkaido University and colleagues developed a novel technique that allowed them to control microtubular deformation and observe its effects on their transportation function.
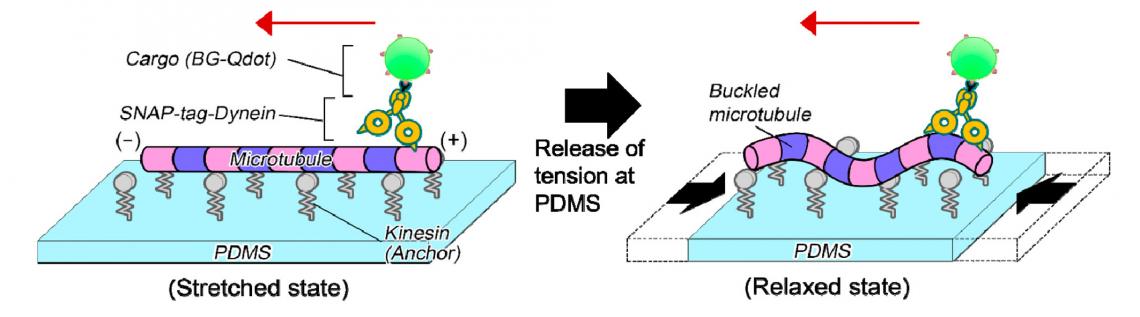
Microtubules were anchored by proteins to an elastic “microstretcher.” When the microstretcher is pulled taut, the microtubules lie parallel to the surface undeformed. Gradually relaxing the microstretcher causes the microtubules to buckle.
The scientists attached motor proteins called dyneins to the microtubules. Inside cells, dyneins appear as if they are walking along microtubules while carrying cargo. In the experiment, the dyneins were conjugated to a fluorescent cargo. The scientists then observed how stretching and relaxing the microstretcher affected dynein-cargo movement along the microtubules.
The newly developed experimental system to analyze dynein-cargo movement along an undeformed (left) and a buckled (right) microtubule on an elastic stretcher composed of polydimethylsiloxane (PDMS). (Syeda Rubaiya Nasrin, Tanjina Afrin, et al., ACS Applied Bio Materials. March 5, 2020)
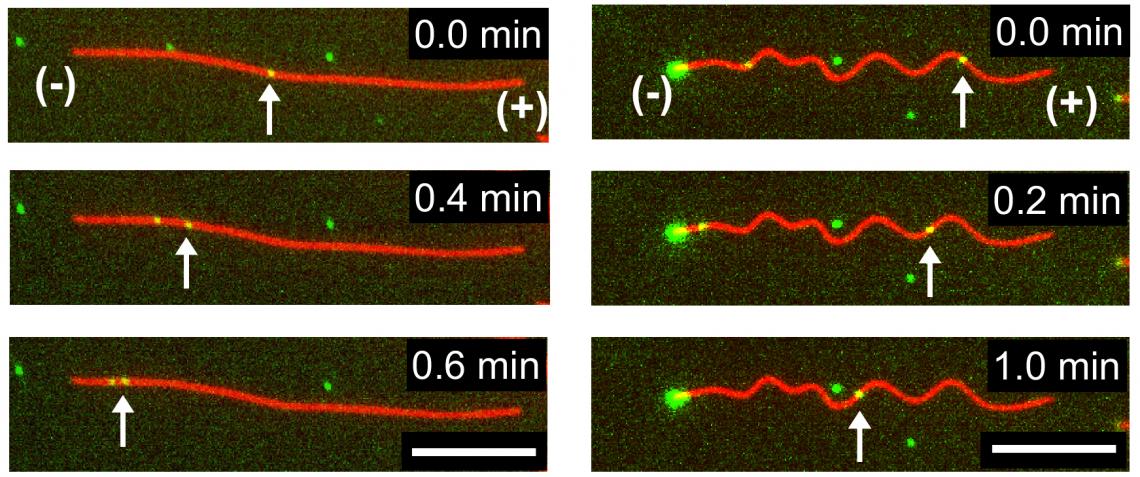
They found the dynein-cargo moved faster as the microtubules began to buckle, but only up to a certain point. Once the buckling strain reached 25%, the moving proteins and their cargo began to slow down. When the microtubules were bent further, they showed complete stops. The scientists also noticed the speed of movement varied along different sections of the buckled microtubules.
Time-lapse fluorescence microscopy images indicating transport of dynein-cargo (green dots marked with arrows) along undeformed (left) and buckled (right) microtubules. (Syeda Rubaiya Nasrin, Tanjina Afrin, et al., ACS Applied Bio Materials. March 5, 2020)
The researchers theorize these altered microtubular dynamics could form the basis of their roles in regulating numerous cellular activities. They next hope to study how microtubular deformation affects the movement of another motor protein, called kinesin, over its surface.
“Our experimental set-up enables us to study the relationship between the deformation of microtubules and their biological functions,” says Kakugo. “The more we understand this process, the closer we might get to designing new nature-inspired materials that can act in a similar way.”
“The findings are also expected to help explain the pathology of traumatic brain injury, which mechanically stresses cells, and neurological conditions like Huntington’s and Parkinson’s diseases, in which microtubules are known to malfunction,” Syeda Rubaiya Nasrin of the research team added.
From the left: Syeda Rubaiya Nasrin, Arif Md. Rashedul Kabir, Akira Kakugo and Kazuhiro Oiwa of the research team.
Contacts:
Associate Professor Akira Kakugo
Material Chemistry Laboratory
Graduate School of Chemical Sciences and Engineering
Hokkaido University
Email: kakugo[at]sci.hokudai.ac.jp
Distinguished Researcher Kazuhiro Oiwa
Advanced ICT Research Institute
National Institute of Information and Communications Technology
Email: oiwa[at]nict.go.jp
Naoki Namba (Media Officer)
Institute for International Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp
Press Office, Public Relations Department
National Institute of Information and Communications Technology
Tel: +81-42-327-6923
E-mail: publicity[at]nict.go.jp