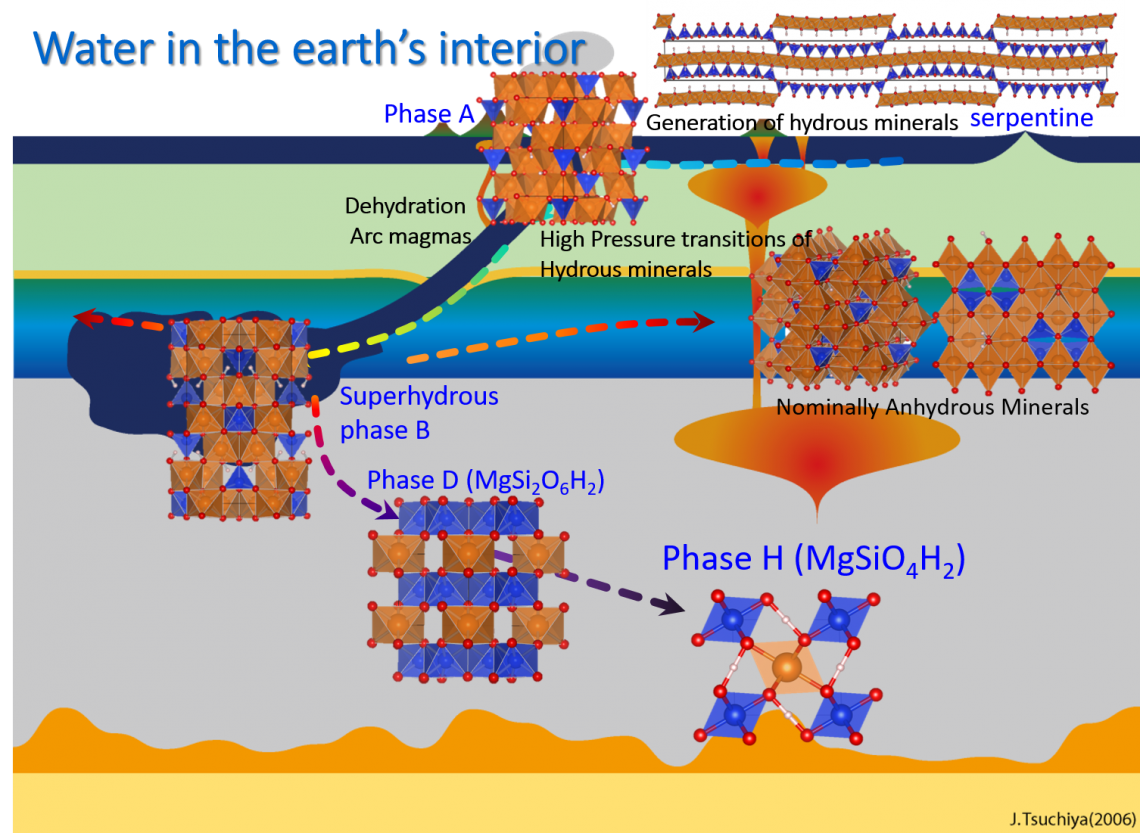
The existence of water in deep Earth is considered to play an important role in geodynamics, because water drastically changes the physical properties of mantle rock, such as melting temperature, electric conductivity, and rheological properties. Water is transported into deep Earth by the hydrous minerals in the subducting cold plates. Hydrous minerals, such as serpentine, mica and clay minerals, contain H2O in the form of hydroxyl (-OH) in the crystal structure. Most of the hydrous minerals decompose into anhydrous minerals and water (H2O) when they are transported into deep Earth, at 40-100 km depth, due to the high temperature and pressure conditions.
However, it has also been reported that some hydrous minerals, called dense hydrous magnesium silicates (DHMSs), may survive in the deeper part of Earth’s interior if the subducting plate is significantly colder than the surrounding mantle. DHMS is a series of hydrous minerals which have high stability under the pressure of deep Earth’s interior. DHMS is also referred to as “alphabet phases”: phase A, phase B, phase D, etc.
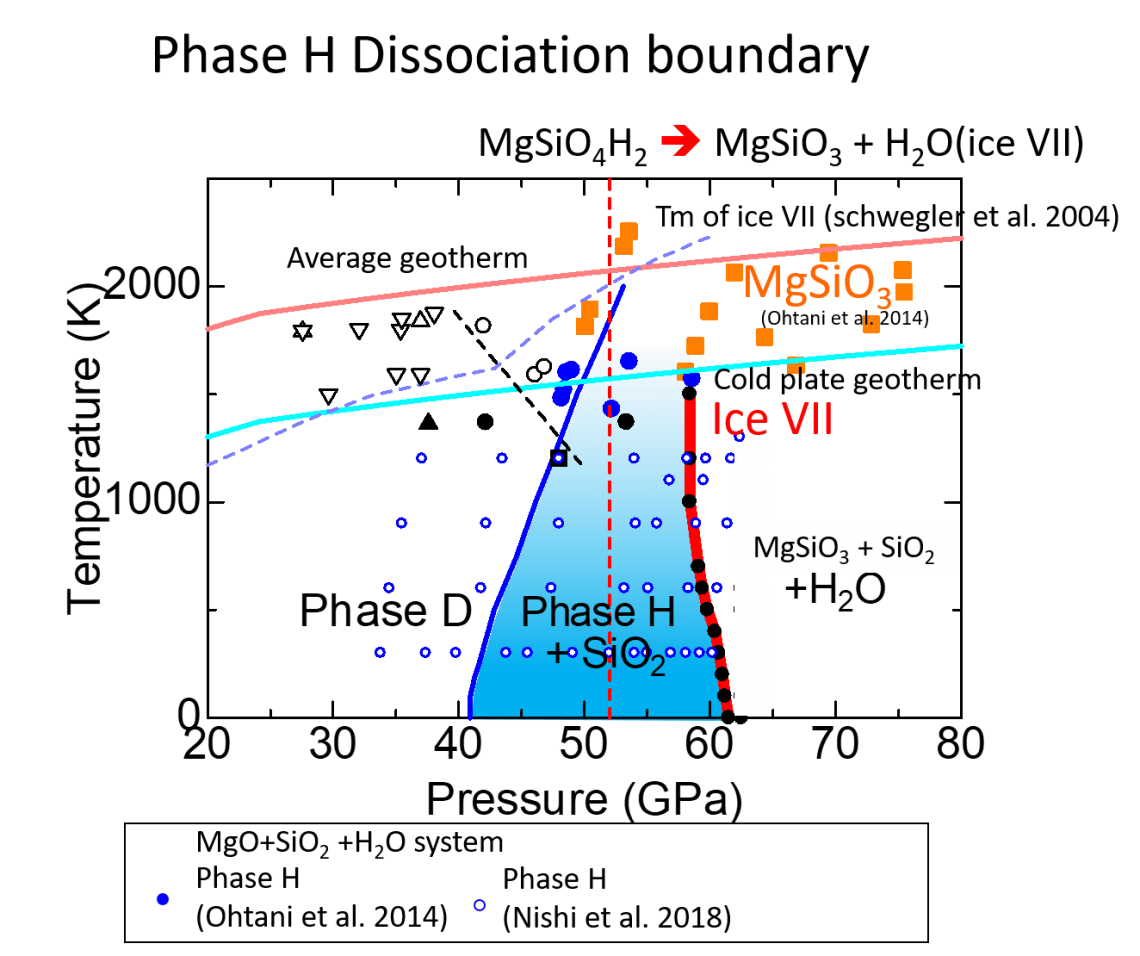
Until recently phase D (chemical composition: MgSi2O6H2) was known to be the highest pressure phase of DHMSs. However, Tsuchiya 2013 conducted first principles calculation (a theoretical calculation method based on quantum mechanics) to investigate the stability of phase D under pressure and found that this phase transforms to a new phase with a chemical composition of MgSiO4H2 (plus stishovite, a high pressure form of SiO2, if the system keeps the same chemical composition) above 40 GPa (GPa=109 Pa). This predicted phase has been experimentally confirmed by Nishi et al. 2014 and named as “phase H” (Figure 1). The theoretical calculation by Tsuchiya 2013 also suggests that phase H finally decomposes into the anhydrous mineral MgSiO3 by releasing H2O by further compression.
Although the theoretical calculation estimated the decomposition pressure of phase H around the middle of the lower mantle (from 660 km to 2900 km depth), a detailed determination has not yet been achieved, because the estimation of the Gibbs free energy of H2O was needed to determine the decomposition pressure of phase H. The Gibbs free energy is a thermodynamic potential that can determine the stability of a system. At lower mantle conditions, the H2O phase has a crystal structure with disordered hydrogen positions, i.e. hydrogen positions are statistically distributed among several different positions. In order to calculate the disordered state of hydrogen, Tsuchiya and Umemoto 2019 calculated several different hydrogen positions and estimated the Gibbs free energy of H2O using a technique based on statistical mechanics.
As a result, they estimated the decomposition pressure of phase H at around 62 GPa at 1000 K, corresponding to the ~1500 km depth (Figure 2). This result indicates that the transportation of water by subducting plate terminates at the middle of the lower mantle in the Mg-Si-O system. Tsuchiya and Umemoto 2019 also suggested that superionic ice may be stabilized by the decomposition of phase H in the subducted plate. In superionic ice, oxygen atoms crystalize at lattice points whereas hydrogen atoms are freely mobile. The chemical reactions between superionic ice and surrounding minerals have not been identified yet, but high diffusivity of hydrogen in superionic ice may produce reactions faster than that in solid ice, but different from water, the liquid phase of H2O.
Reference
J. Tsuchiya (2013) First principles prediction of a new high pressure phase of dense hydrous magnesium silicates in the lower mantle, Geophysical Research Letters, 40, 4570-4573, doi:10.1002/grl.50875.
M. Nishi, T. Irifune, J. Tsuchiya, Y. Tange, Y. Nishihara, K. Fujino, Y. Higo (2014) Stability of hydrous silicate at high pressures and water transport to the deep lower mantle, Nature Geoscience, 7, 224-227, doi:10.1038/ngeo2074.
J. Tsuchiya, and K. Umemoto (2019) First-principles determination of the dissociation phase boundary of phase H MgSiO4H2, Geophysical Research Letters, 46, 7333-7336, doi:10.1029/2019GL083472
Water is transported into Earth’s deep interior by dense hydrous magnesium silicates (DHMSs).
The thick red line indicates the calculated dissociation phase boundary of phase H.