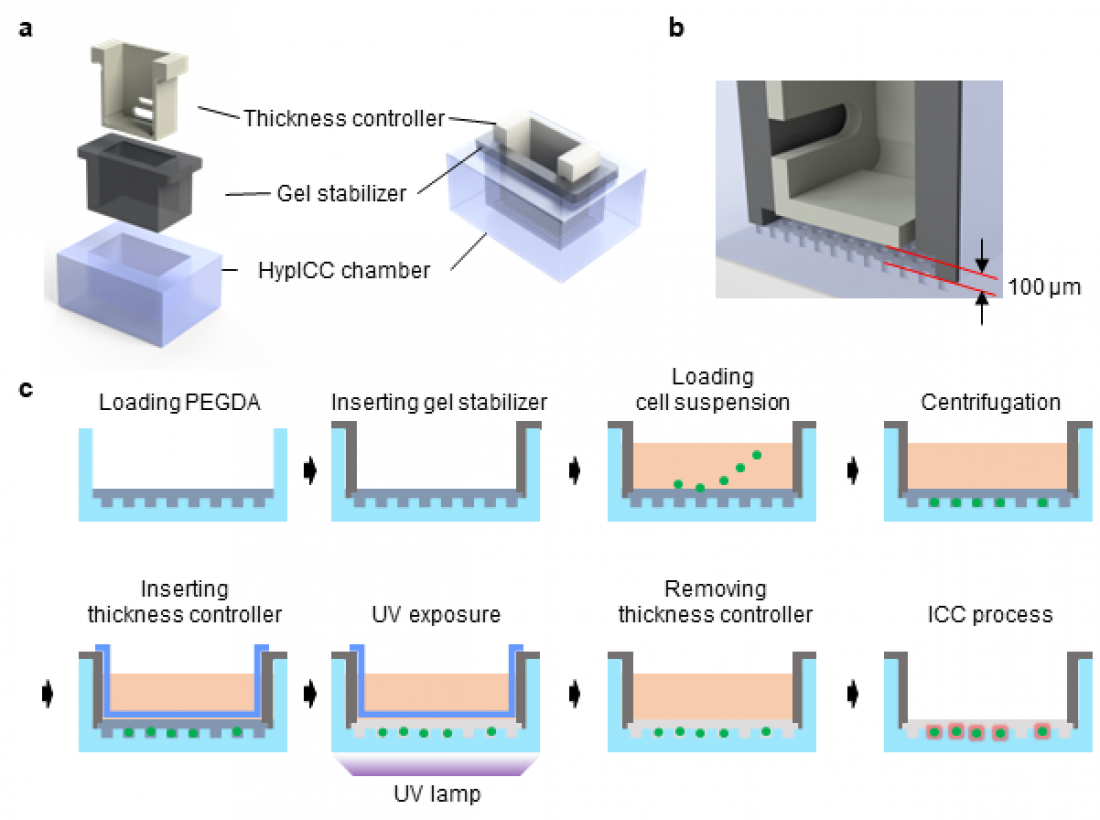
□ DGIST (President Yang Kook) Professor Minseok Kim of the Department of Neurobiology and his team developed lossless immunocytochemistry technology, which facilitates analysis of rare cells that present in trace amounts in clinical specimens. The corresponding technology developed together with CTCELLS, Inc. involves the use of an ultra-thin film hydrogel to facilitate fluid exchange while inhibiting cell loss, and a higher preservation rate and reproducibility were achieved compared to existing cell staining technologies. Rare cells could be analyzed without loss; thus, this technology could be applied in future clinical research.
□ Recently, various anticancer drugs have been developed, and the potential for cancer treatment is improving; however, attaining accurate information on cancers that frequently modify or spread remains difficult. Currently, methods for identifying genetic information from cancer include tissue biopsies using cancerous tissues for diagnosis and liquid biopsies using blood. The liquid biopsy method is more efficient. It can identify information about a cancer through non-invasive inspection that does not cause pain, and a more accurate diagnosis is possible when circulating tumor cells (CTCs) are used because the genetic information for all cancers is available. Aside from cancer diagnosis, when an embryo cell within a pregnant woman’s blood is separated during prenatal diagnosis, non-invasive prenatal diagnosis can be achieved with a higher accuracy. Thus, technology for analysis of rare cells within a clinical specimen is a significant foundation for future diagnosis technology.
□ However, CTCs or embryo cells are present in trace amounts within the blood; thus, analysis is significantly difficult. Because this amount is in a few milliliters of blood, the use of existing technologies could result in errors caused by loss or cell deformation during analysis. Thus, accurate information is difficult to obtain.
□ Thus, Professor Minseok Kim’s research team developed ‘ultra-thin-film hydrogel control technology’ that fundamentally obstructs cell loss that may occur during cell capturing or cell staining, and the morphological modification of cells was minimized. This technology showed a significantly high (97% or above) cell preservation rate compared to existing technologies, and the clinical utility was identified through joint research with Professor Soyeon Oh’s team at Pusan National University Yangsan Hospital and Professor Youngjoon Kim’s team at Samsung Changwon Medical Center. Additionally, its utility in various rare cell analyses, such as circulating embryo cell detection for prenatal diagnosis, was identified through joint research with Professor Hyunmi Ryu’s team at Cha University Bundang Medical Center.
□ DGIST Professor Minseok Kim of the Department of Neurobiology stated that “the developed lossless immunocytochemistry technology is element technology in the field of rare cell-based diagnosis, which is significant for the capacity to increase diagnosis accuracy,” and revealed that “it is hoped that this technology will improve early cancer diagnosis and prenatal diagnosis accuracy and be utilized in various rare cell-based clinical specimen analyses.”
□ Meanwhile, this research was proceeded as part of a project at the Basic Research Laboratory of the research foundation, the results were published in March in ACS Applied Materials & Interfaces, and the study was selected as a cover paper. The corresponding technology was transferred to CTCELLS, Inc., and commercialization is in progress.
Correspondence Author Email Address : [email protected]