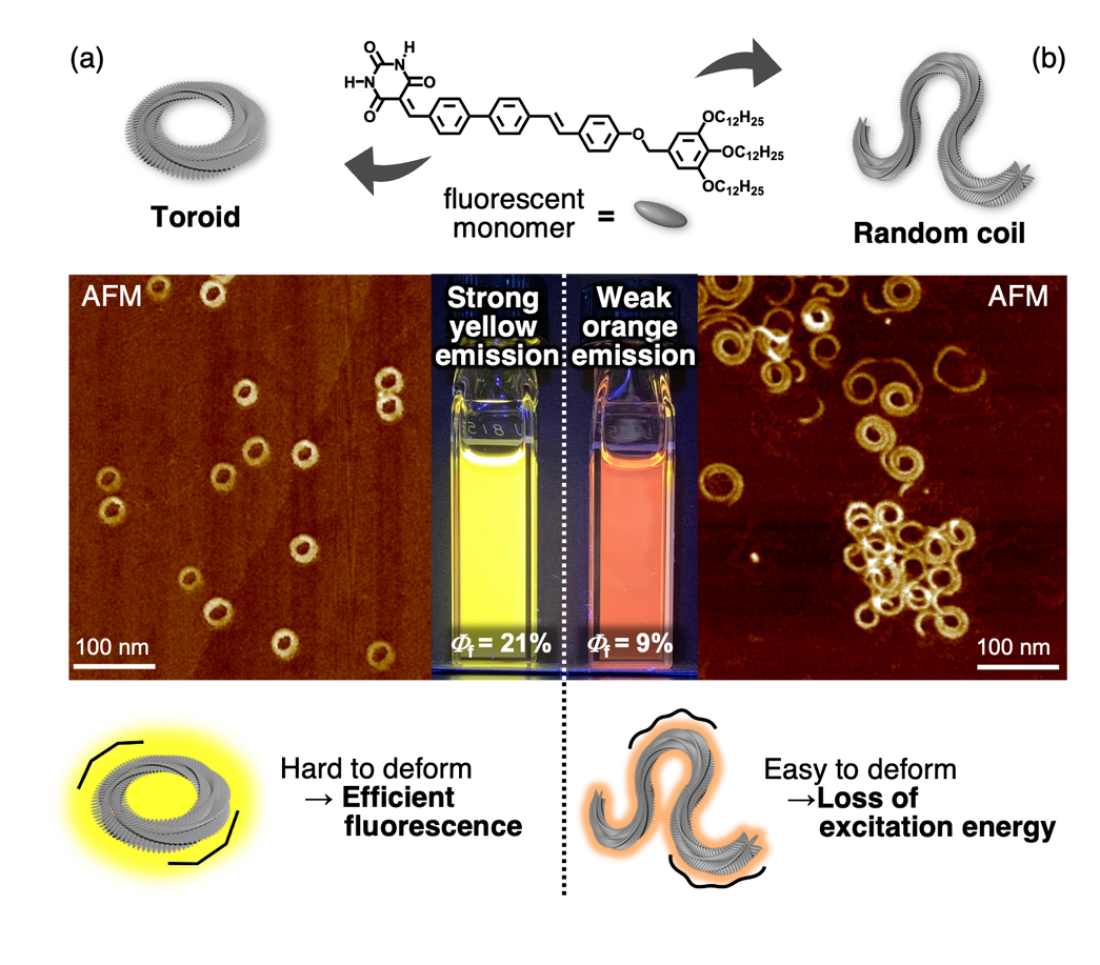
Fig. Structure-dependent fluorescence properties of molecular assemblies.
(a) The toroidal assemblies with no termini are not easily deformed in solution, resulting in less excitation energy loss and strong yellow fluorescence. (b) The randomly coiled assemblies are easily deformed, resulting in excitation energy loss and a weak orange fluorescence.
Scientists explore the photoluminescence properties of toroidal and randomly coiled supramolecular polymers
In supramolecular chemistry, the self-assembly state of molecules plays a significant role in determining their tangible properties. Controlling the self-assembled state has garnered significant attention as it can be exploited to design materials with desired properties like charge transport capability and fluorescence wavelength. For years, scientists have been trying to decipher how molecular organization impacts the properties of supramolecular assemblies that are in the nano (<10 nm) and mesoscopic (10–1000 nm) scales. However, the study of structures with supramolecular polymer assemblies derived from the same monomer is often hindered by dynamic structural changes and immature control over self-assemblies.
A recent study published on January 1, 2024, in the Journal of the American Chemical Society, investigated the properties of one-dimensional mesoscale supramolecular assemblies of two different structures composed of the same luminescent molecule. It showed how two structures showed very different properties depending on whether they had their molecules arranged in a closed circular pattern or not. The study was led by Prof. Shiki Yagai from Chiba University, with Sho Takahashi, a doctoral course student at the Graduate School of Science and Engineering at Chiba University, as the first author. It also included Prof. Martin Vacha from the Department of Materials Science and Engineering at Tokyo Institute of Technology, and Dr. Hikaru Sotome from the Graduate School of Engineering Science at Osaka University as corresponding authors.
"The geometrical beauty of a circular structure, which has no termini and no corners, has fascinated people. Chemists have realized the synthesis of giant cyclic molecules using various approaches not only to create beautiful structures but also to compete in the elegance of the process of synthesizing such beautiful structures," says Prof. Yagai, speaking of the inspiration behind this study. "The best example of nature utilizing the functional beauty of circular structures would be the light-harvesting antenna organ (LH2, LH1) of purple photosynthetic bacteria. LH2 has a beautiful circular structure due to the protein's outstanding self-organizing ability, and it is thought that by arranging chlorophyll dyes in a circular array based on this framework, lean light collection and excitation energy transfer are achieved."
Through the self-assembly of luminescent molecules synthesized based on their own molecular design, the team obtained a mixture of two one-dimensional π‑conjugated molecular aggregates with different structures, namely terminus-free cyclic structures (toroids) and randomly coiled structures. The mixture exhibited low-energy and low-intensity luminescence.
The two structures were separated using a novel dialysis technique that exploited the difference in their kinetic stability. Post-separation, it was shown that the terminus-free closed toroidal structure led to higher energy and more efficient luminescence when compared to random coils. The team carried out ultrafast laser spectroscopy to investigate the mechanism of their topology-dependent fluorescence properties. The results indicated random coils with termini lost excitation energy due to defects generated by fluctuations in solution, unlike toroids that were not easily deformed and exhibited fluorescence without energy loss. Furthermore, it was found that in the mixed solution of toroids and random coils, the excitation energy was transferred from the toroid to the random coil due to the agglomeration of both assemblies, and only the random coil-derived luminescence was observed.
This study establishes morphological control of materials at the mesoscale as a possible new guideline for the design of functional materials. It also highlights that in the case of materials that are prone to supramolecular polymorphism, such as the toroid and random coil, it is essential to purify the assemblies before analyzing their photophysical properties. If not separated, the results obtained might only reflect biased properties instead of distinct ones due to energy transfer between different structures.
The researchers are hopeful that these insights can encourage the development of high-performance flexible devices using cyclic molecular assemblies. "We can gladly say that a correlation between structural beauty and functional beauty has been found here, even in meso-scale molecular assemblies. We believe that the insights from our study could help improve the performance of solar cell devices and light-emitting devices in the long run, thereby facilitating their widespread acceptability and enriching people's lives along the way," concludes Prof. Yagai.
###
The article, “Impact of Ring-Closing on the Photophysical Properties of One-Dimensional π‑Conjugated Molecular Aggregate,” was published in Journal of the American Chemical Society at DOI: https://doi.org/10.1021/jacs.3c11407
About Professor Shiki Yagai
Shiki Yagai is a Professor at the Division of Advanced Science and Engineering, Graduate School of Science and Engineering, Chiba University, Chiba, Japan. In 2002, he received his PhD from Ritsumeikan University, Japan. He joined Chiba University as an assistant professor and was promoted to full professor in 2017. He has over 160 publications in the fields of organic chemistry, supramolecular chemistry, and nanotechnology, with more than 7500 citations. He has been bestowed with several awards, the most recent being the Swiss Chemical Society Lectureships (2017). Currently, Prof. Yagai and his team are working on the development of supramolecular functional materials.
About Chiba University
Chiba University boasts 10 faculties and 17 graduate schools on 5 campuses and a rich academic environment where students can acquire a broad-based interdisciplinary education as well as an advanced level of expertise.While respecting diversity in learning, Chiba University promotes innovative research through collaboration and researcher support programs leading to the development of new fields of research, which will continue to make a wide range of social contributions both locally and internationally.
https://www.chiba-u.ac.jp/e/index.html
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
https://www.titech.ac.jp/english/
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and is now one of Japan's leading comprehensive universities with a broad disciplinary spectrum. This strength is coupled with a singular drive for innovation that extends throughout the scientific process, from fundamental research to the creation of applied technology with positive economic impacts. Its commitment to innovation has been recognized in Japan and around the world, being named Japan's most innovative university in 2015 (Reuters 2015 Top 100) and one of the most innovative institutions in the world in 2017 (Innovative Universities and the Nature Index Innovation 2017). Now, Osaka University is leveraging its role as a Designated National University Corporation selected by the Ministry of Education, Culture, Sports, Science and Technology to contribute to innovation for human welfare, sustainable development of society, and social transformation.
Website: https://resou.osaka-u.ac.jp/en