Tokyo, Japan – Optimum performance of upcoming thermochemical energy storage and other technologies depends on proper applications of the principles that underpin salt ion hydration. Now, researchers from Japan have solved a long-standing puzzle of ion hydration that will be useful in electrolyte design for such technologies.
In a study recently published in Nature Communications, researchers from the Institute of Industrial Science, The University of Tokyo have proposed an explanation for the ion-specific properties of ion hydration in water-based solutions. By revealing the nature of ion hydration and how it can be dramatically stabilized, they have advanced a broad spectrum of applications across various fields, such as chemistry, biology, and materials science.
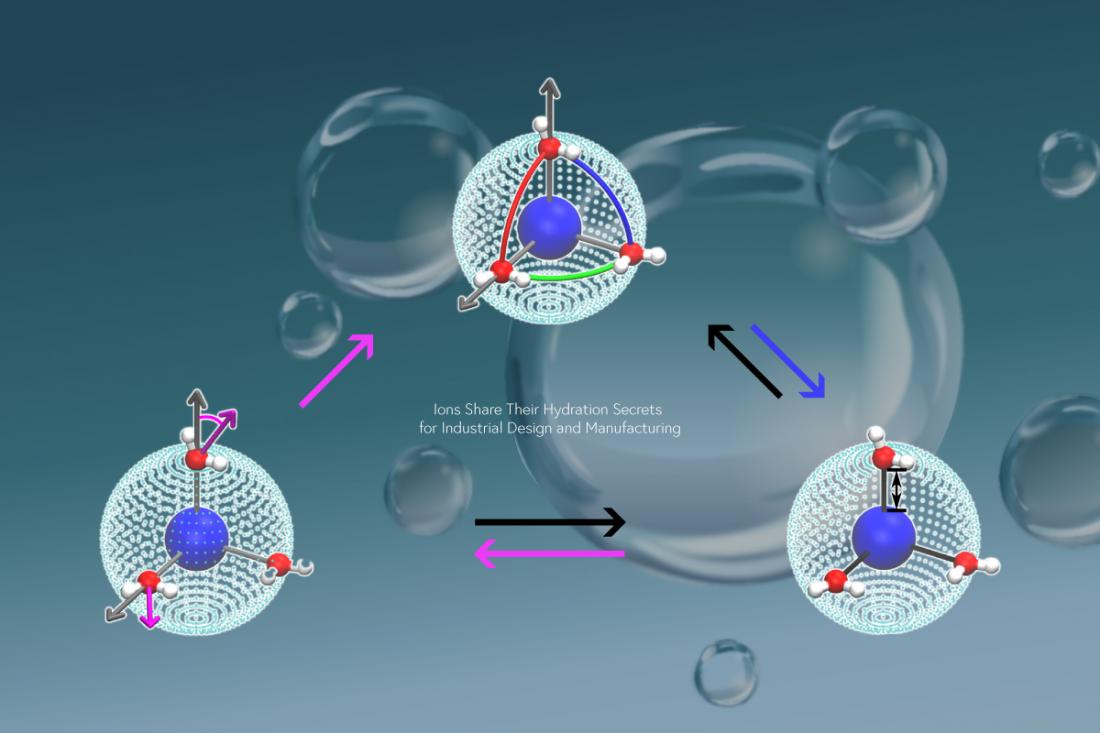
To explain salt ion hydration, researchers have long grouped such ions into those that are structure-forming (facilitate formation of water molecule networks) and structure-breaking (disrupt these networks). However, recent evidence that this explanation is too simplistic suggests the need for a new molecular-level explanation of ion hydration. Deriving such an explanation has been attempted in prior work by focusing on the ionic field, which depends on the relationship between the ionic charge and the ion–water distance, as a possible driving force for ion hydration. These investigations have focused on ions with discrete sizes and charges that are present in nature, rather than on synthetic or unnatural ions.
Corresponding studies that also include unnatural salt ions with continuous size and charge would greatly aid in developing a comprehensive understanding of the ionic field. Obtaining such understanding by computational simulations is the problem that the researchers sought to address.
“Our molecular dynamics simulations focus on pseudo-main-group cations that interact with water through two intermolecular forces, Coulomb and Van der Waals interactions,” explains Rui Shi, lead author. “Our work reveals previously unknown details of the ion hydration shell and their impact on water dynamics.”
The researchers’ main result is that ions with a lower charge density interact with more water molecules, which undergo weak bonding with each other. This result is general, regardless of the ion. The distance at which the ion–water interaction is as strong as water–water hydrogen bonding can be viewed as the crossover between accelerating versus decelerating water dynamics around the ion.
“We also reveal an explanation for the 11 orders of magnitude difference in the residence times of hydration water,” says Hajime Tanaka, senior author. “By tuning the ion size and charge to specific combinations, we realize the bond-orientational order of hydrated water molecules can develop and stabilize the hydration shell. This leads us to establish a new mechanism for the highly stable hydration of certain ions.”
This work has broad applications across multiple disciplines, including chemistry, biology, materials science, and various industries. For example, research on using salt hydrates for energy storage and purification technologies for RNA-based medical therapeutics will benefit from modern insights into the principles that underpin ion hydration in water-based media.
###
The article, “Impact of hierarchical water dipole orderings on the dynamics of aqueous salt solutions,” was published in Nature Communications at DOI: 10.1038/s41467-023-40278-x.
About Institute of Industrial Sciene, The University of Tokyo
The Institute of Industrial Science, The University of Tokyo (UTokyo-IIS) is one of the largest university-attached research institutes in Japan. UTokyo-IIS is comprised of over 120 research laboratories—each headed by a faculty member—and has over 1,200 members (approximately 400 staff and 800 students) actively engaged in education and research. Its activities cover almost all areas of engineering. Since its foundation in 1949, UTokyo-IIS has worked to bridge the huge gaps that exist between academic disciplines and real-world applications.