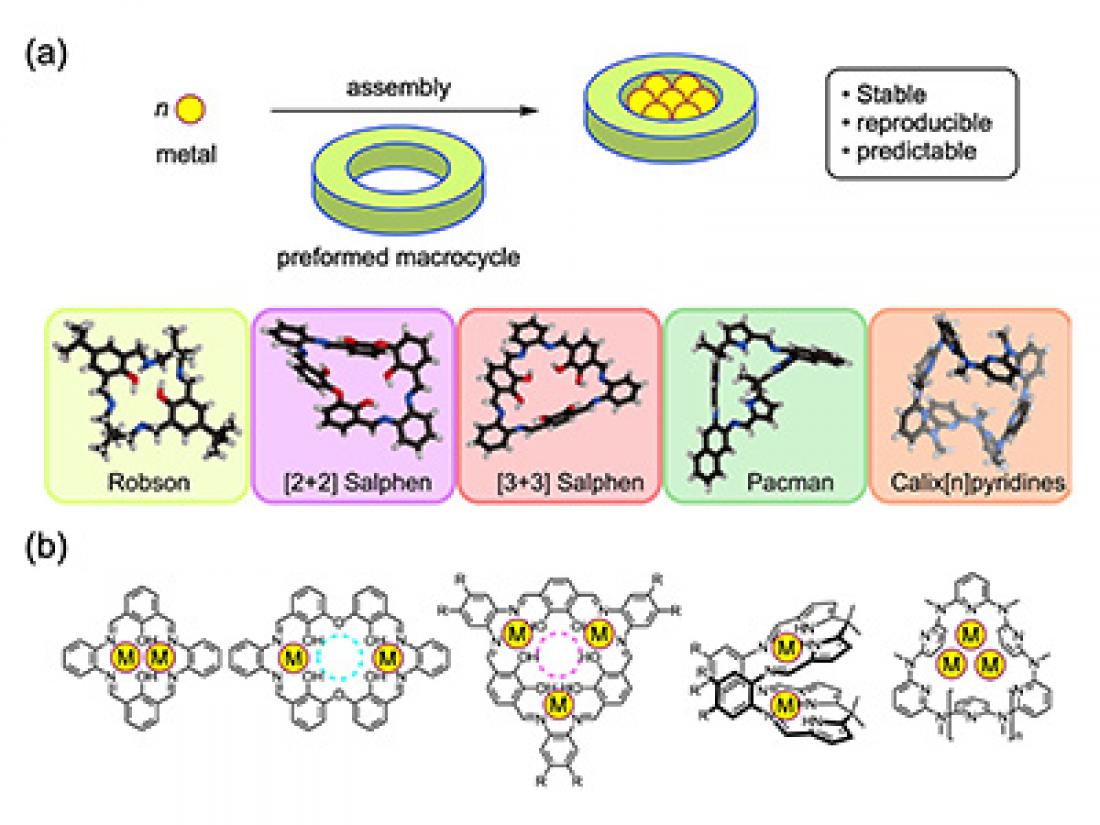
Contemporary macrocycles used for the synthesis of discrete polymetallic complexes: (a) concept, (b) chemical structures of representative polymetallic complexes.
In polymetallic complexes, two or more metal atoms combine with organic molecules into larger, complicated molecular structures. Such complexes are used in the development of e.g. new catalysts, molecular magnets and sensors. In the past, polymetallic complexes were often synthesized by the trial-and-error method of mixing metal ions with organic ligands, resulting in unpredictable compounds. The modern approach involves macrocycles: organic molecules having a ring-structure. The inside space of macrocyclic molecules can be used to anchor a polymetallic complex during its formation, a ‘trick’ that enables the reproducible synthesis of predictable end products. Shigehisa Akine from Kanazawa University, Mark MacLachlan from the University of British Columbia (UBC) and NanoLSI (Kanazawa University), and UBC PhD student Mohammad Chaudhry have now published a comprehensive overview of the synthesis of polymetallic complexes via the macrocycle route, which also discusses how certain properties of a complex can be tuned by changing the composition of the macrocycle used.
The scientists first discuss the origins of the field. In the 1970s, it was shown that so-called [2+2] macrocycle dinuclear complexes could be formed by using a relatively simple organic compound, with molecular formula C9H8O3, as the building block. These dinuclear complexes consist of two metal atoms sitting in an organic ‘web’ with 2-fold symmetry. Similar Robson macrocycles, as they are called, can be obtained with 6 metals, with the overall [3+3] structure having 3-fold (triangular) symmetry. Robson macrocycles are still researched today, but the method remains somewhat unpredictable.
The researchers then explain how [2+2] (with two-fold symmetry) and [3+3] (with triangular symmetry) macrocycles also feature in contemporary designs. The [3+3] compounds are nowadays actively researched because of their potential as single-molecule magnets — molecules that exhibit (para)magnetism. Using macrocycles, the magnetic properties of the ensuing molecules can be tuned by changing cluster size and composition. Regarding the [2+2] complexes, these are noted to have cavities that can be exploited for creating unique clusters.
Another interesting class of multimetallic structures are the ‘Pacman macrocycles’, built from ligands displaying a cleft. This geometry can be used to capture and activate small metal–ligand–metal molecules. In this context, Pacman macrocycles with two uranium atoms have been intensively studied in the context of nuclear waste processing. Akine, MacLachlan and Chaudhry also show that, more generally, by using Pacman ligands, chemists have succeeded in making several structurally and chemically unique polymetallic complexes.
The final type of macrocycles discussed by the researchers features pyridine rings (pyridine is similar to benzene, with one C–H unit replaced by nitrogen). Pyridine-ring macrocycles offer high flexibility, and can be used to synthesize a variety of complicated multimetallic structures — the authors give plenty of examples of silver-containing complexes.
The scientists finish their review with an outlook of this fascinating research area. Specifically, they note that a future trend is likely to be the mimicking of the activity of naturally occurring clusters in living systems. Indeed, polymetallic complexes play key roles in important reactions such as the reduction of nitrogen to ammonia and the oxidation of carbon monoxide to carbon dioxide. Quoting Chaudhry et al.: “The potential selectivity and control rendered by the macrocycle-templating method is especially attractive for consistent, reproducible production of functional multimetallic clusters.”