This research was co-led by Dr Youngjin Lee from the Department of Neuroscience (NS) and Professor Lawrence Wu from the Department of Materials Science and Engineering (MSE) at CityU. Their findings were published in the scientific journal Science Advances, titled “Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma”.
Metabolic characteristics in cancer cells
A hallmark in the malignant progression of glioma cells is the metabolic reprogramming for their survival. Compared to normal cells, cancer cells can live without oxygen. Moreover, they prefer using glycolysis, a metabolic process which converts glucose into high-energy molecules without the need of oxygen, even in conditions with normal oxygen level. Hence tumour glycolysis is often called “aerobic glycolysis”, or “Warburg’s effect”, to distinguish from the normal anaerobic glycolysis of healthy cells.
In glycolysis, malignant glioma cells produce a tremendous amount of lactates that are harmful to tumour cells. The tumour cells thus significantly enhance the levels of a major lactate transporter, monocarboxylate transporter 1 (MCT1), and its binding protein, CD147, to remove the lactate out of cells for the maintenance of continuous glycolysis for producing energy and survival. Therefore, many studies proposed to use MCT1 and CD147 in the glioma cells as a biomarker for the diagnosis and treatment of malignant glioma.
“While most of the researches have focused on the glioma cells or the genes, we are more interested in the interactions between glioma cells and their surrounding cells via exosomes,” said Dr Lee who is a neuroscientist.
Exosomes are nanovesicles with diameters of 30-200 nm released by glioma cells. They contain tumour-specific messenger RNA (mRNA) and mutant proteins and promote tumour progression by transporting these pro-oncogenic molecules to neighbouring cells. Also, they can cross the blood-brain-barrier and the blood-cerebrospinal fluid barrier, meaning that they can be detected in the blood.
A new biomarker in exosomes
In the study, the team discovered that MCT1 and CD147 in glioma cells actually controlled the release and composition of the exosomes. “This means that knocking down the MCT1 and CD147 can produce an anti-cancer effect not only via inhibiting the progression of cancer cells themselves. But it also blocks the interaction of cancer cells and surrounding cells via exosomes, including the transport of pro-oncogenic molecules to other cells,” said Dr Abhimanyu Thakur, first author of the paper and research assistant from NS.
More importantly, the team found that there are also MCT1 and CD147 inside exosomes. And the MCT1 and CD147 levels in exosomes increased during the hypoxia-driven malignant progression of glioma cells. The quantitative analysis further showed that the MCT1 and CD147 levels in exosomes could reflect the respective levels in glioma cells. This means the exosomal MCT1 and CD147 can also be used as a biomarker for tracking the malignant progression of glioma.
Microscope images of exosomes (in green colour). Fig A shows the normoxic environment while Fig B shows the hypoxic environment. More exosomes are found in the hypoxic environment. (Photo source: DOI number: 10.1126/sciadv.aaz6119)
Non-invasive and label-free detection
Then how to measure the MCT1 and CD147 levels in exosomes?
The current standard tools for diagnosing and tracking the progression of glioma are magnetic resonance imaging (MRI) and computed tomography (CT) scans, as well as needle biopsies in brains. But MRI and CT scans cannot make detections at the molecular level due to limited resolution and primarily only determine later stages of glioma, while biopsies are invasive.
By using the localized surface plasmon resonance (LSPR) and atomic force microscopy (AFM) biosensors modified by Professor Wu and his PhD students, the team successfully achieved a precise quantitative detection of the MCT1 and CD147 levels in exosomes released by the glioma cells.
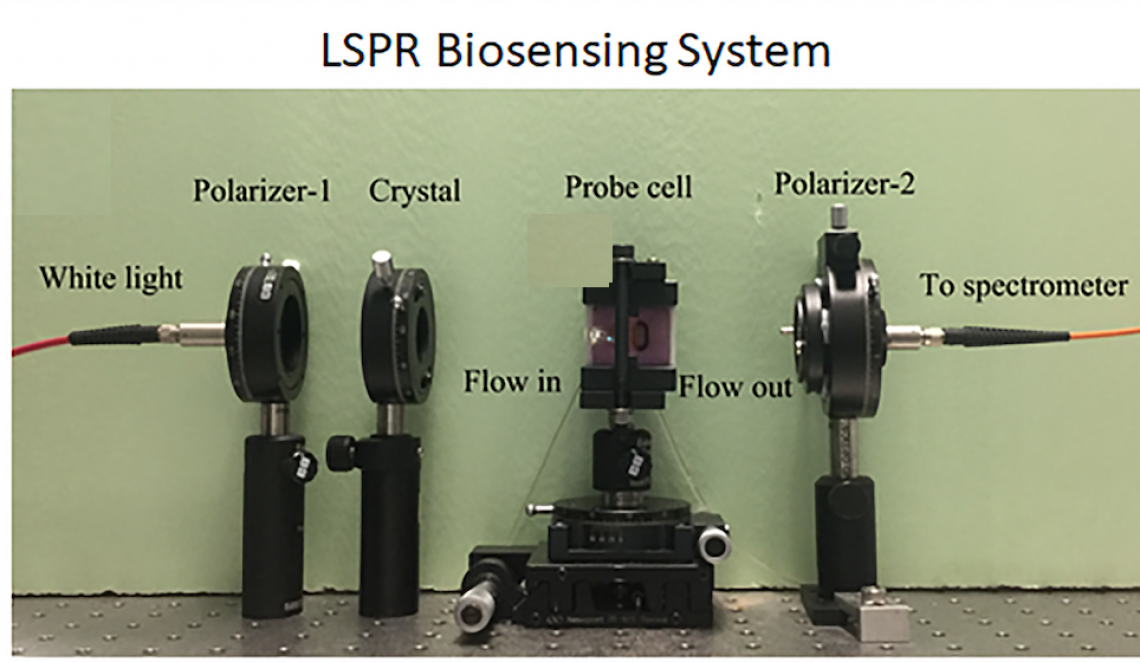
This is the LSPR biosensing system used by the team. (Photo source: City University of Hong Kong)
LSPR is a technique with high sensitivity for detecting various single molecular interactions, such as antigen-antibody interaction or protein-protein interaction. It works by detecting changes in the refractive index of the testing solutions at the surface of a sensor. AFM is a scanning probe microscope with high resolution (up to fractions of a nanometre) for analyzing biological samples, including exosomes.
Both LSPR and AFM are cost-effective, real-time, highly sensitive and “label-free”, meaning that contrast agents are not needed. “Labelling process takes a lot of efforts and is expensive. With label-free biosensing tools, we do not need to label the exosomes but can still detect them,” explained Professor Wu.
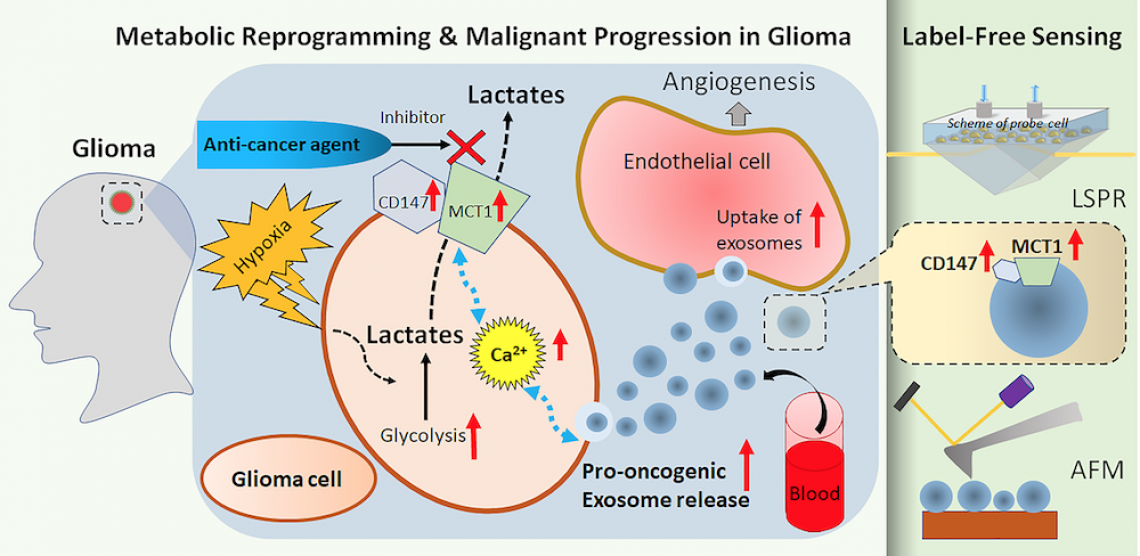
Hypoxic glioma cells significantly increase the release of pro-oncogenic exosomes containing a high level of MCT1 and CD147. The enhanced exosomal MCT1 and CD147 from malignant glioma cells is precisely detected by label-free LSPR and AFM biosensors. (Photo source: Dr Abhimanyu Thakur & Xu Chen)
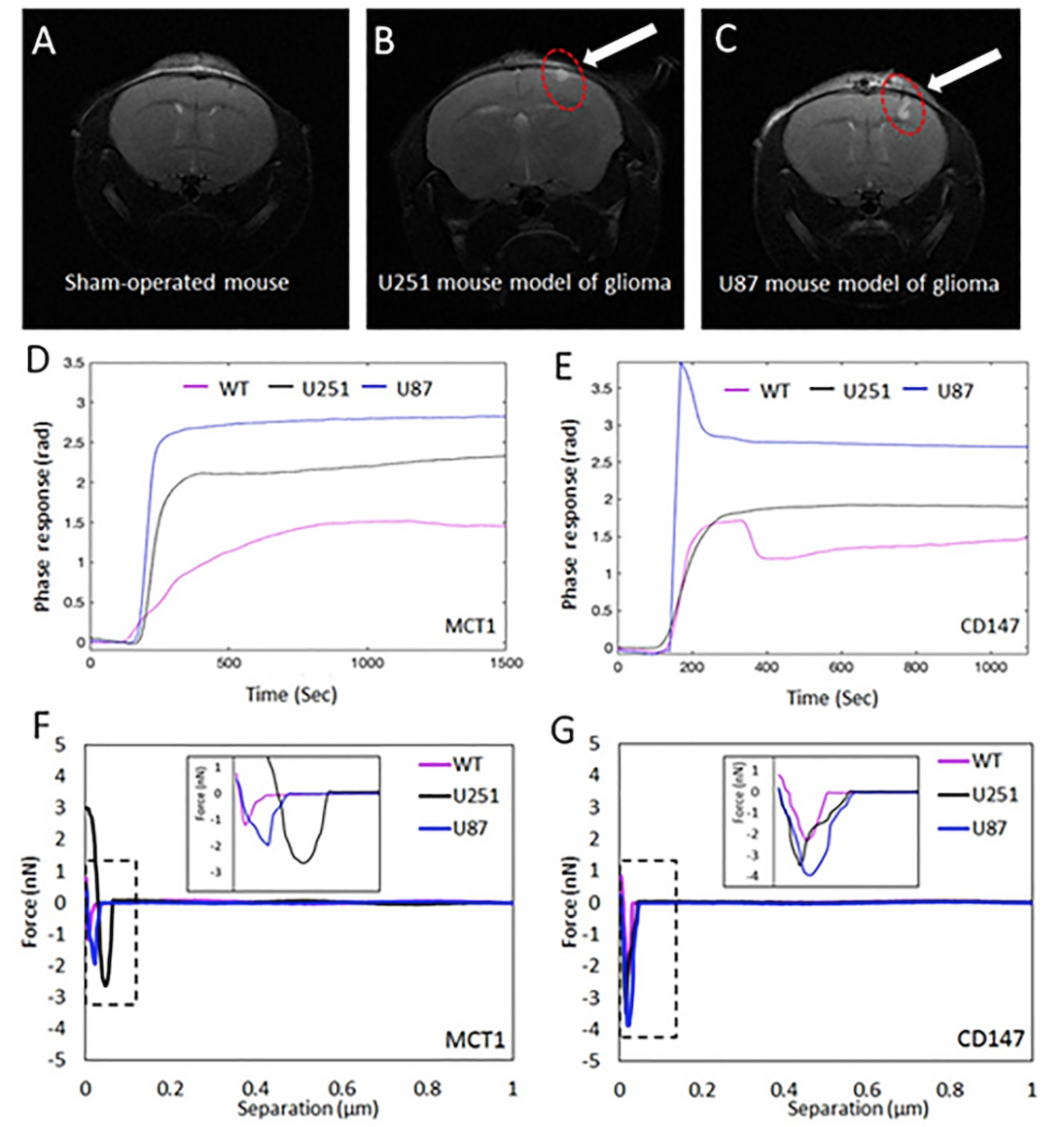
The top row shows MRI images of the mouse without brain tumour (fig A) and mouse with brain tumour (red circle in both fig B and C). Fig D and E show the phase responses detected by the LSPR biosensor. WT refers to mouse without brain tumour while U251, as well as U87 are mouse with brain tumour. Fig F and G show the force curves of the AFM biosensor. Both LSPR and AFM responses show MCT1 and CD147 in blood serum derived exosomes from a mouse with glioma were significantly greater compared to those from healthy mice. (Photo source: DOI number: 10.1126/sciadv.aaz6119)
His team modified the LSPR and AFM by putting a special coating of gold nanoislands (small nanoparticles) on the surface of the sensors. “This helps to achieve higher sensitivity in LSPR and higher resolution in AFM,” explained Xu Chen, Professor Wu’s PhD student participated in the research.
To test the performance of the modified LSPR and AFM biosensors, they conducted experiments using exosomes released from cultured human glioma cells and the blood serum of mouse with glioma respectively. The positive results strongly support the application potential of LSPR and AFM biosensors to monitor the malignant progression of glioma cells, for early detection and better diagnosis of the disease.
Application potential in neurological diseases
“Our study indicated that metabolic changes in neural cells could facilitate disease progression by altering exosomal interactions. It will be interesting to further study the correlation between metabolic reprogramming and altered exosome communication in neurological diseases,” Dr Lee added. “The detection of exosomal biomarkers using label-free LSPR and AFM biosensors can be potentially applicable to the diagnosis and prognosis of other neurological diseases, such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.”
All the research team members are from CityU. Apart from Dr Lee, Professor Wu, Dr Thakur, and Mr Xu, other research team members included Yang Tian from the Department of Neuroscience Department, Dr Qiu Guangyu and Ng Siu-pang from the MSE department, as well as Dr Kannie Chan Wai-yan and Han Xiongqi from the Department of Biomedical Engineering.
The study was supported by CityU and the University Grants Committee.
DOI number: 10.1126/sciadv.aaz6119