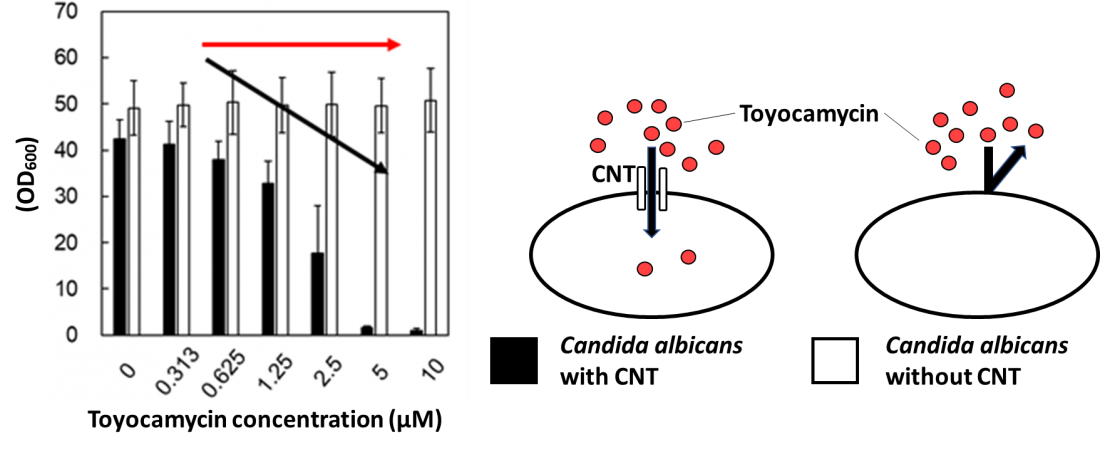
CNT makes Candida albicans sensitive to toyocamycin. Cell proliferation between parental and CNT-deficient strains of C. albicans exposed to different concentrations of toyocamycin (left). At higher concentrations toyocamycin kills C. albicans, entering the cells through CNT in the cell membrane, without CNT present C. albicans is not sensitive to toyocamycin (right).
Mycosis is an infectious disease caused by parasitic mold or yeast and treated with an antifungal, a subgroup of antibiotics. However, fungi—like humans—are eukaryotes, so creating new antifungal drugs that are toxic to fungi while being safe for people is a difficult problem that Associate Professor Yoshihiro Ojima and Professor Masayuki Azuma, at the Osaka Metropolitan University Graduate School of Engineering, are trying to solve. They focused on toyocamycin, an antibiotic known to work against the pathogenic yeast Candida albicans but does not kill baker's yeast (Saccharomyces cerevisiae). Candida albicans causes candidiasis; however, toyocamycin is also toxic to humans, so it is not used to treat Candida albicans infections.
“Antifungal agents effective against pathogenic eukaryotes are more difficult to develop than antimicrobial agents; there are only about 10 drugs in four systems available for treatment,” explained Professor Azuma.
The researchers were intrigued because S. cerevisiae does not have a concentrative nucleoside transporter (CNT) gene, while humans and C. albicans do. They suspected that the reason toyocamycin is effective against C. albicans but not S. cerevisiae is that it is transported into C. albicans by the CNT protein. So, they tested toyocamycin on C. albicans that had the CNT gene disrupted by genome editing.
Losing the CNT gene rendered toyocamycin ineffective against C. albicans, indicating that toyocamycin is taken into the cells by the CNT protein. They also found that S. cerevisiae became sensitive to toyocamycin when human CNT3 was introduced into cells by gene recombination.
The uptake of toyocamycin and its analogs by the C. albicans and the human CNT3 proteins was tested by introducing the genes into S. cerevisiae. Testing of toyocamycin and analogs revealed that their uptake varied, depending on the structure and shape of the molecule.
“This discovery, learning how different CNT proteins transport toyocamycin into cells, may lead to the development of new systems of antifungal drugs with unprecedented modes of action,” said Professor Ojima.
By focusing on structural differences between analogs, new antifungal drugs could be developed that are effective only against C. albicans and harmless to humans, to treat candidiasis or other mycosis in the future.
###
About OMU
Osaka Metropolitan University is a new public university established by a merger between Osaka City University and Osaka Prefecture University in April 2022. For more science news, see https://www.upc-osaka.ac.jp/new-univ/en-research/, and follow @OsakaMetUniv_en, or search #OMUScience.