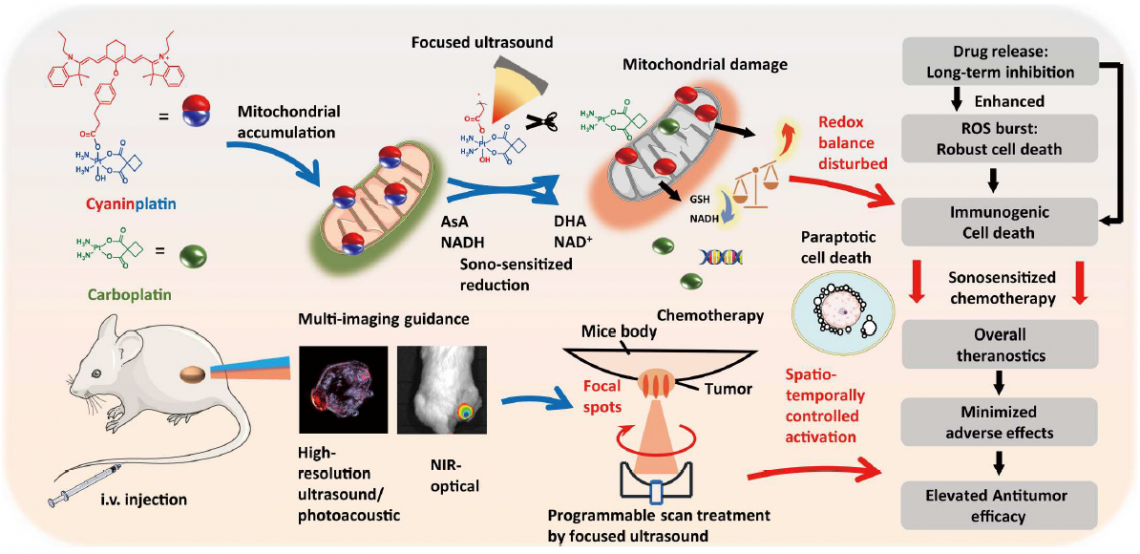
Schematic illustration of the working mechanism of cyaninplatin, a small-molecule Pt(IV) anti-cancer prodrug, which is activated by focused ultrasound and reduced to chemotherapeutic carboplatin after irradiation. (Credit: Liu, G. et al., source: https://www.science.org/doi/10.1126/sciadv.adg5964)
“Photoactivated chemotherapy has successfully enhanced treatment by providing location-specific therapy with reduced adverse effects,” said Professor Zhu Guangyu in the Department of Chemistry at CityU. “However, its shallow penetration depth and strong optical scattering have yet to meet the need for a non-invasive therapy capable of precise ablation of deep tumours.”
To overcome this limitation, the research team, led by Professor Zhu and Professor Wang Lidai, Associate Professor in the Department of Biomedical Engineering (BME), invented the SSCT technique by developing cyaninplatin, a sono-activatable anticancer prodrug, and tailor-making a programmable focused ultrasound system (FUS) for sono-activation.
Cyaninplatin is a small molecule platinum(IV) anticancer prodrug (a prodrug is a pharmacologically inactive compound before activation inside the body) with a modified ultrasound-responsive, theranostic (a combination of diagnosis and therapeutics) platinum (Pt) coordination compound, and a carboplatin-based Pt(IV) scaffold. It accumulates well in the tumour region and when irradiated with ultrasound, it is reduced to carboplatin, which is a typical chemotherapy drug. When activated by focused ultrasound with spatiotemporal control, it can focus directly on cancer cells.
In their experiments, the team found that the ultrasound-activated cyaninplatin sufficiently induced cancer cell oxidation, eventually leading to mitochondrial DNA damage and cancer cell death.
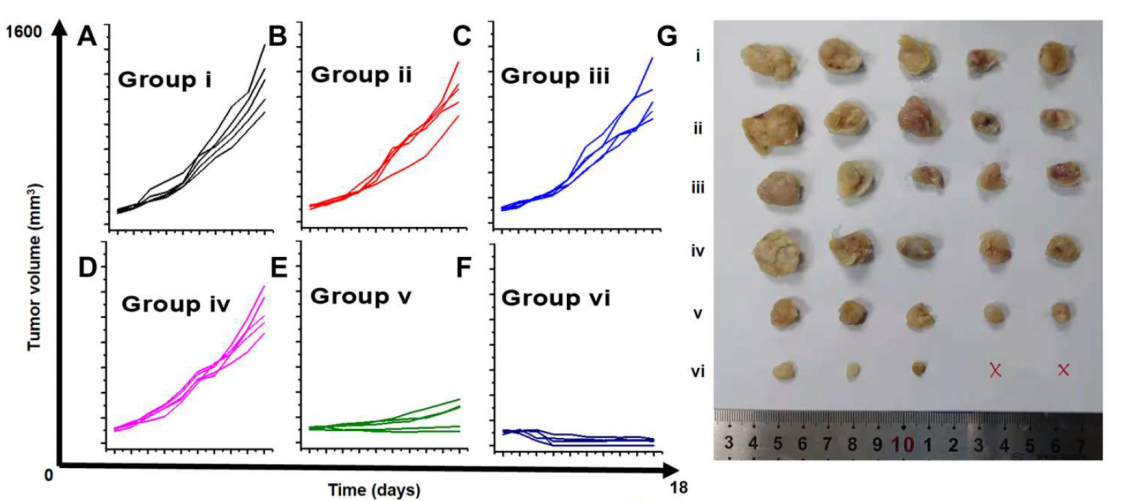
The ultrasound-activated cyaninplatin drastically inhibited the growth of tumours and completely eradicated two tumours (group vi).
(Credit: Liu, G. et al., source: https://www.science.org/doi/10.1126/sciadv.adg5964)
“Another fascinating part of the research is that we successfully utilised ultrasound’s property of deep penetration in tissues for the activation of small-molecule drugs,” said Professor Wang. Previous research reported that photoactivation with near-infrared light was effective only in the millimetre range and performed poorly when the cancer cell was covered with thick body tissue. But combined with the customised FUS, the treatment with ultrasound-activated cyaninplatin successfully inhibited cancer cell viability by 51% with 1cm-thick tissue coverage and 33% with 2cm-thick coverage.
“Our well-designed FUS enables the SSCT to be a precise tumour-specific treatment with good penetration performance,” said Professor Wang. “More importantly, our system allows the ultrasound to focus on a specific area within 8mm, and hence highly focuses the ultrasound energy on a tiny area to activate sono-responsive prodrugs, providing an efficient approach with minimal side effects.”
In addition, the prodrug’s fluorescence property enables it to act as a multi-imaging contrast agent, allowing the visualising and reconstructing of a tumour in a semi-3D fashion. This provides accurate guidance for applying FUS at a tumour site and monitoring the drug-accumulation time.
The cyaninplatin is a chemical complex with a modified ultrasound-responsive theranostic Pt-coordination compound and carboplatin-based Pt(IV) scaffold. (Credit: City University of Hong Kong)
To further explore the efficacy of SSCT, the team injected inactivated cyaninplatin into mice with tumours. After the fourth treatment on day 6, the ultrasound-activated cyaninplatin drastically inhibited the growth of tumours and completely eradicated two tumours. In contrast, there was no inhibition of tumour growth for the untreated groups; the tumours continued to grow.
The ultrasound-activated cyaninplatin also lowered the level of the tumour proliferation factor to 24%, preventing potential tumour recurrence. And there was no notable loss of body weight, no abnormal behaviours, and no systemic toxicity to the main organs of the treated mice.
“The ultrasound-activatable prodrug can be further extended to preclinical or clinical studies for cancer treatment,” said Professor Zhu. “The findings in this study may provide an important reference for developing novel therapeutic approaches, providing new dimensions for anticancer treatment and broadening the field of medical ultrasound applications.”
The findings were published in the scientific journal Science Advances under the title “An Ultrasound-Activatable Platinum Prodrug for Sono-Sensitized Chemotherapy”.
Professor Zhu Guangyu (second from left) and Professor Wang Lidai (second from right) and their research team, including Mr Liu Gongyuan (first from right) and Miss Chen Shu (first from left), at CityU. (Credit: City University of Hong Kong)
The study’s co-first authors are Mr Liu Gongyuan, a PhD student in the Department of Chemistry and Dr Zhang Yachao, former postdoc in the BME at CityU. The corresponding authors are Professor Zhu and Professor Wang.
This research is supported by Hong Kong Research Grants Council, the National Natural Science Foundation of China, and the Science Technology and Innovation Committee of Shenzhen Municipality.