Professor Jaeheung Cho (left) and MS-PhD Student Donghyun Jeong (right)
A research team led by Professor Jaeheung Cho of Emerging Materials Science discovered manganese-iodosylbenzene compound, which is new active intermediate, from the high-efficient oxidation of biomimetic catalyst and artificial oxidants that imitated in vivo enzyme catalysts, and clarified the oxidation mechanism. In vivo reactions usually occur as various enzymes inside human body act together. ‘Metal enzyme’, which especially has certain metal elements among enzyme, must form ‘metal-active oxygens’ by combining with oxygen for in vivo oxidation. For more efficient oxidation, related areas employ iodosylbenzene, an artificial oxidant to help facilitate the combination of oxygen and enzyme.
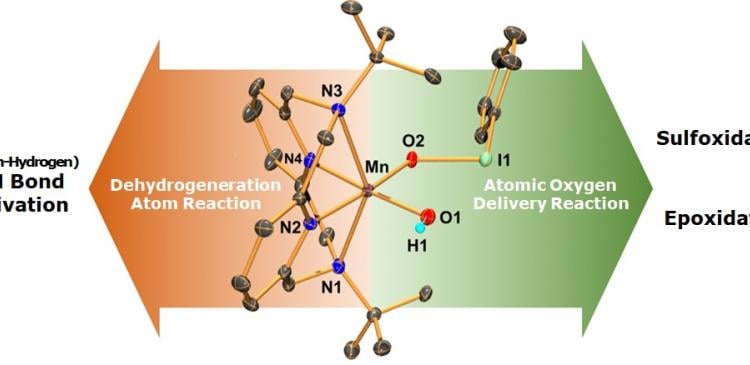
However, it has been continuously observed that artificial oxidants are combined with the metal elements of metal enzyme, forming metal-iodosylbenzene before oxygen is transferred to metal enzyme. Thus, there have been many debates among scientists on how much metal-iodosylbenzene influences the oxidation of enzyme and oxygen. To clarify this, Professor Cho’s team started conducting a synthesis research on iodosyl-benzene, an artificial oxidant, and biomimetic manganese complex, which imitated manganese center among in vivo metal enzyme.
During the research, Professor Jaeheung Cho’s team found intermediates where trivalence manganese-iodosylbenzenes are combined, which has never been discovered before. The team identified the structure by analyzing single crystals and even proved oxidation mechanism as well. Professor Cho said “The big significance of this research is that it discovered trivalence manganese-iodosylbenzene that has never been discovered before and clarified the formation process. While there is still a long way to go until realizing its commercialization, I expect to be able to make contributions to the development of enzyme reaction research by continuously researching reaction mechanism to improve the efficiency of enzyme, which acts as a catalyst in oxidation.”
The study has been published on ‘Journal of the American Chemical Society (JACS),’ the top chemistry academic journal, on November 28. Dong-hyeon Jung, an Integrated MS-PhD degree student in the Department of Emerging Materials Science at DGIST, participated in the research as the 1st author.
For more information, contact:
Jaeheung Cho
Associate Professor
Department of Emerging Materials Science
Daegu Gyeongbuk Institute of Science and Technology (DGIST)
E-mail: [email protected]