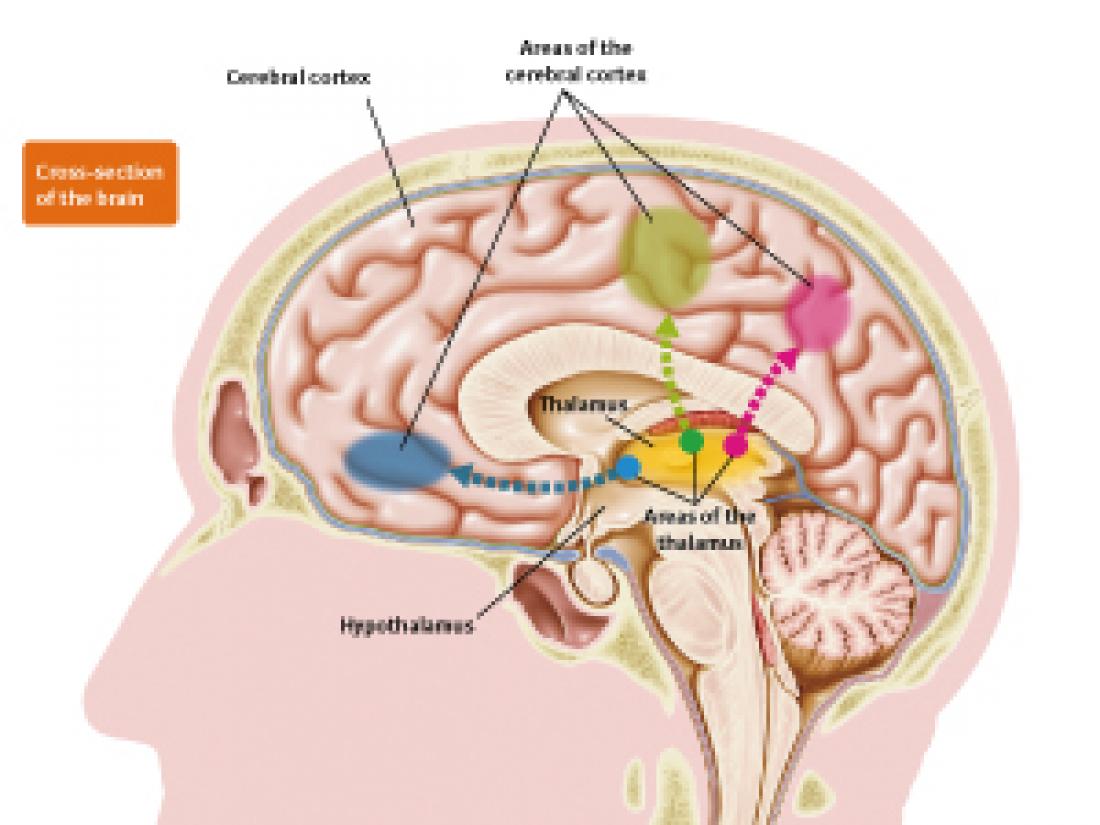
Connection between areas of the thalamus and respective areas of the cerebral cortex.
The thalamus has a different area for each kind of information to be processed. Dendrites of neurons in the thalamus elongate, connect to each area of the cerebral cortex, and transmit information.
Tomomi Shimogori
Unit Leader
Shimogori Research Unit
RIKEN Brain Science Institute
What is the difference between humans and animals? For example, it is impossible for mice or dogs to speak with human words, not only because the shape of their mouths or vocal cords is different, but also because their cerebral cortex lacks the speech area that processes complex language. “We want to know how new areas such as the speech area formed in the cerebral cortex of humans in the process of evolution,” says Tomomi Shimogori, unit leader of the Shimogori Research Unit. She and her colleagues are trying to understand the mechanism that creates new areas in the brain and to understand the functions that exist only in the human brain. In the future, this research is expected to contribute to our understanding of the causes of diseases such as autism, which are thought to be rooted in the initial development process of the brain, and to develop better treatments.
The research papers on developmental biology were boring
The cerebral cortex covers the surface of the human brain and is patterned with many grooves. “If the cerebral cortex were stretched and spread out, it would be about the size of a newspaper sheet,” says Shimogori. “If we continue with the newspaper example, the cerebral cortex has a different area for each kind of information processed, like the economics page, the local news page and the weather page. The cerebral cortex has not only become larger in humans than in mice or dogs, but has developed many new areas that process various kinds of information. Humans have thus acquired a high degree of knowledge and language ability.”
However, whether in mice, dogs or humans, the first stage of brain development is the formation of neural tubes in the cells of the first layer of the cerebral cortex. These neural tubes thicken and transform into complex shapes to form the brain. The areas of the cerebral cortex are formed completely when an organism is born. The cerebral cortex is large and has many areas in humans but is small and has only a few areas in dogs and mice because of differences in the development processes of the brain.
Shimogori, who previously researched cancer and other diseases in a pharmacology department, began researching brain development after she was appointed to a postdoctoral position at the University of Chicago in the US in 1998. “The development of the brains of mouse fetuses is very beautiful to watch. The neural tubes thicken and change shape before your very eyes, the cells migrate to each location, and the dendrites elongate and connect accurately to other cells. Then they begin to function as a brain. I thought it was such a beautiful and mysterious phenomenon. But the research papers on developmental biology that were being published at that time were all about research using knockout mice, and none of those papers were interesting.”
In the 1990s, development biology was thriving with research on the functions of genes during the developmental process. In these studies, ‘knockout mice’ were created by destroying specific genes, and the treated mice were compared with normal mice. However, the method used in those days for making knockout mice was problematic in that the functions of the specific genes were lost in all of the cells from the beginning stage of development.
For example, it has been pointed out that the fibroblast growth factor FGF8 may perform important functions during the formation of the cerebral cortex. However, if the gene that makes FGF8 is destroyed in the beginning stage of development, the fetus will die before the cerebral cortex is formed. “FGF8 performs important functions that are essential to the development of not only the brain but also various other regions of the body. Consequently, it was not possible to study how FGF8 functioned in the cerebral cortex by the methods used in those days to create knockout mice.”
Controlling areas of the cerebral cortex
“If methods can be developed for manipulating the activity of genes during specific periods and in specific regions, I think more interesting research will be possible.” Shimogori took notice of a gene transfer technique called ‘electroporation’, which was being applied to chicken fetuses. Electroporation is a method of injecting genes directly into specific regions of embryos and introducing the genes into cells by applying electrical stimulation. Using electroporation, it is possible to exclusively overexpress specific genes during specific periods, or to inject other genes to inhibit the activity of specific genes. “My goal was to apply this method to mice.”
In chickens, it is relatively easy to perform embryo manipulation, such as the introduction of genes, because development occurs within the eggshell. However, this operation is difficult in mice because development occurs in the uterus. It is necessary to remove the uterus with the fetus from the belly of an anesthetized mother mouse, introduce a gene into the fetus, and put the fetus back into the mother’s body. “I made my own electrodes for applying electrical stimulation, and through trial and error I eventually succeeded in establishing a method for introducing genes into mice.”
Using this method for introducing genes, Shimogori manipulated expression of FGF8 in the cerebral cortex of mice and studied how changes occurred in the way that areas were formed within it.
As a result, when she overexpressed FGF8, an area called the ‘barrel field’, which processes sensory information from the whiskers, moved behind its original position, and the motor area and frontal lobe that had formed in front of it expanded. When she introduced a gene that inhibited the activity of FGF8, on the other hand, the barrel field moved forward and the visual field that had formed behind it expanded. “FGF8 is secreted from the front of the region that becomes the cerebral cortex. Once the FGF8 is secreted, its concentration in the cerebral cortex becomes higher on the front side and lower on the back side. This difference in concentrations may cause differences in which genes are expressed and thus may cause different areas to form in the front and back areas of the cerebral cortex.”
In addition, when Shimogori expressed FGF8 in the back side of the cerebral cortex, where it was not originally expressed, a new barrel field formed. “By changing the way that FGF8 is expressed, I was able to change the position and size of the areas of the cerebral cortex. If we put this in terms of a newspaper, I enlarged the weather page and created an additional economics page. But I was unable to create a new area.”
Focusing on the thalamus, which inputs information into the cerebral cortex
How did new areas form in the cerebral cortex during the process of human evolution?
“Although new areas may be formed in the cerebral cortex, they do not function unless they are connected to other areas. For example, the visual area requires input of visual information that is received from the outside world through the eyes. Except for smell, sensory information from the outside world such as sight, hearing, touch and taste is collected in the thalamus and sent to the cerebral cortex. “I thought that the reason new areas form in the cerebral cortex during evolution might be that changes in the habitat environment cause new information to enter from the thalamus.”
In 2004, Shimogori launched a research unit at the RIKEN Brain Science Institute and started full-blown research on the thalamus. “The thalamus is difficult to study because it is in the depths of the brain. Consequently, thalamus research is not progressing as fast as research on other regions of the brain. Also, the number of thalamus researchers is very small.”
The thalamus is divided into areas according to the information being processed. The dendrites of the neurons in each of these areas elongate, connect to their respective areas in the cerebral cortex, and transmit information to those areas. The size and number of areas in the thalamus are known to differ among animals. So how are new areas formed in the thalamus? “To answer that question, it is first necessary to investigate how the thalamus is formed and how each area is formed within it. During the development process, cells change in shape and properties, and move great distances, so we follow this phenomenon while extrapolating what kinds of cells these cells will become. For this purpose, landmarks are necessary.”
Those landmarks are the genes that are expressed in the thalamus. Shimogori and her colleagues discovered at least 1,000 kinds of genes that are expressed in the mouse thalamus and studied when and where their expression began and how their patterns of expression changed. “If we can understand which genes are expressed exclusively in each area, we can enhance and suppress the expression of those genes and study what kind of effect this enhancement and suppression has on the formation of areas in the cerebral cortex, which is an access point.”
For example, in the thalamus of higher animals, there is a particular area called the ‘pulvinar’, which does not exist in rodents such as mice. In humans, part of this pulvinar is highly developed and is connected to the lateral prefrontal cortex area of the frontal lobe, which exists only in the cerebral cortex of primates. Accordingly, if it is known which genes are necessary for pulvinar formation, the way that they influence formation of the frontal lobe can be investigated.
Did a new way of using genes cause the brain to evolve?
To deduce the evolution of the cerebral cortex from the thalamus, it is necessary to study and compare the formation process of the thalamus in various species of animals. Shimogori and her colleagues are now investigating how genes corresponding to genes discovered in the mouse thalamus are expressed in the chicken thalamus.
If the differences in gene expression between mice, which are mammals, and chickens, which are birds, can be compared, the characteristics of thalamus formation in mammals should become apparent.
Through collaborative research with Norihiro Okada and his colleagues from the Tokyo Institute of Technology, Shimogori and her colleagues have determined that whereas FGF8 is expressed strongly in the hypothalamus of mice, it is hardly expressed at all in the hypothalamus of chickens.
DNA fragments called ‘retrotransposons’ are what enhance expression of FGF8 in the hypothalamus of mice. Retrotransposons make copies of themselves and enter other locations in the genome (total genetic information). When a retrotransposon enters a switch region that controls the expression of genes, it can sometimes enhance and suppress that expression. In mice, retrotransposons enter the genetic switch of FGF8 and enhance its expression in the thalamus during development.
The mapping of genomes of various animals has progressed remarkably in recent years and has led to the discovery that all animals are very similar in terms of the number of genes that they have and in the chemical substances and base sequences that constitute their DNA, even when the animals differ greatly in abilities and form. So, what causes differences in abilities and form among animals? Researchers are beginning to think that these differences are caused by differences in the time, location and strength of their gene expression, namely, by differences in the way that genes are expressed. One of the main causes of these differences in the use of genes is retrotransposons. The genomes of mammals contain many retrotransposons, which in humans make up 40% of the genome. They are thought to be deeply involved in the evolution of mammal brains.
So what happens when FGF8 expression in the mouse thalamus is enhanced by retrotransposons? Shimogori and her colleagues discovered that when FGF8 expression is further enhanced by a gene transfer method, areas inside the thalamus move behind their original position. In contrast, if the activity of FGF8 is suppressed, the areas move to the front of their original position. FGF8 plays an important role in forming areas in the thalamus in the same way that it does in the cerebral cortex.
In contrast, FGF8 is hardly expressed at all during thalamus formation in chickens. What differences does FGF8 cause in the formation of mammal and bird brains? “We can explore that question by injecting FGF8 into the thalamus of a chicken during its development. I want to conduct that experiment in the future.”
Did the function of sorting information in the thalamus cause the evolution of the cerebral cortex?
Shimogori points out the importance of figuring out the unknown functions of the thalamus and simultaneously exploring the process of thalamus formation. “The thalamus may have acquired the ability to sort information during evolution. This may have caused the evolution of the cerebral cortex.”
For example, the thalamus developed the ability to sort information about the outline, color and depth of objects from visual information and send it to different locations in the cerebral cortex. New areas may have developed in the cerebral cortex as a result. “Among primates who lived in treetops, some may have emerged that acquired a new area in the brain that enabled stereoscopic vision by extracting depth information in the thalamus and sending it to the cerebral cortex. Thus, probably only the monkeys who had acquired advanced stereoscopic vision were able to move around efficiently from tree to tree and quickly find fruit, thus winning the struggle for survival.”
Expanding a research network based on a research foundation
Through collaborative research with Johns Hopkins University in the US, Shimogori and her colleagues are preparing a database that will show the time and location of the beginning of expression of genes that function in the development of the thalamus. It will also show changes in the expression patterns of these genes. The information will be displayed in the form of stereoscopic images, and Shimogori and her colleagues are already preparing to release this database on the Internet. This will be the first thalamus database of its kind. “Five years have passed since this research unit was launched at RIKEN, but during that time, we have mainly been collecting tools for researching the thalamus. Some might think, ‘You are still collecting tools after five years?’ But in order to understand the complex network that is the brain, a solid research foundation is essential. It is also necessary to promote collaborative research with researchers from various fields and expand this research network based on this foundation.”
Full-blown research can then be pursued on deducing the evolution of the cerebral cortex from the thalamus. In the future, this research is also expected to contribute to medicine. “For example, conditions such as autism in humans may be caused by errors in the formation of specific areas or nerve connections in the brain. Knowing what areas exist in the human brain and how neurons are connected in order to function properly might be useful for understanding the causes of diseases such as autism, and for developing treatments for them.”
About the Researcher
Tomomi Shimogori
Tomomi Shimogori was born in Chiba, Japan, in 1970. She graduated from the Hoshi Collage of Pharmacy, Tokyo, in 1993, and obtained her PhD in pharmaceutical science from Chiba University in 1998. After six years of postdoctoral training at the Department of Neurobiology, University of Chicago, USA, she returned to Japan to join the RIKEN Brain Science Institute as unit leader exploring the mechanism of thalamic development and its contribution to cortical evolution. Her research focuses on the developmental mechanisms patterning the mouse thalamus, comparative analysis of developing mouse and chick thalamus, and the role of thalamocortical axons in cortical plate development.