Institute of Bioengineering and Nanotechnology, A*STAR
Singapore, July 28, 2011 – The future of regenerative medicine lies in harnessing the potential of the human body to renew and repair itself. Now, scientists at the Institute of Bioengineering and Nanotechnology (IBN), the world’s first bioengineering and nanotechnology research institute, have developed a new genetic engineering technique that promises safer stem cell therapy for cancer patients. Using an insect virus, the team of researchers successfully inserted a therapeutic gene into a safe site in the DNA of human embryonic stem cells without compromising the functionality of the engineered cells.
Human embryonic stem cells (hESCs) and induced pluripotent stem (iPS) cells have the unique ability to transform themselves into any cell type, making them attractive candidates for cell-based therapies in regenerative medicine. Many medical applications require the integration of therapeutic genes into these stem cells before they are transplanted into the patient. However, current genetic engineering techniques lack accuracy and are not effective enough in ensuring that the therapeutic genes would function properly after they are integrated into chromosomes of the stem cells.
The most commonly used method of introducing transgenes into hESCs is random integration. This is a technique that could result in unwanted mutations, and possibly even tumor formation of the transplanted cells. It would also be difficult to predict whether the randomly inserted transgene would be able to function properly, as certain DNA sequences may trigger a malfunction or completely ‘shut down’ the gene. Therefore, there is a compelling need to develop a new technique that provides better control over mthe precise location of gene integration without affecting the genomic stability.
Previous studies have identified the adeno-associated virus integration site 1 locus (AAVS1) as a ‘safe harbor’ for the addition of a new gene into the human genome as there is no known adverse effect on the cell resulting from gene disruption at this site, and the inserted gene has been shown to retain its competence across diverse cell types. Two known methods of delivering genes directly into AAVS1 utilize the AAV2 technology and the zinc-finger nuclease technology. The former has a very low targeting efficiency rate of 4%, indicating a very high risk of random gene insertion. Despite the
improved targeting efficiency of the latter (33-61%), it could be toxic to cells and not ideal for use in vivo.
According to IBN Group Leader, Dr Shu Wang, “Having observed the technical complications that plague cell-based therapy, we decided to find a way to enhance the safety and unearth the true potential of this form of therapy for disease treatment. Our lab has been developing stem cell-based vehicles for cancer therapy over the last few years, and we needed a safe method to introduce tumor-killing therapeutic genes into stem cells.”
IBN’s technique has been shown to achieve up to 100% targeting accuracy, and the IBN researchers are the first to demonstrate homology recombination at the AAVS1 site in hES cells, and to combine the use of baculoviral vectors with the Cre/loxP recombinase system to target and splice specific DNA sequences in these cells.
“As baculoviruses are insect viruses that are known not to insert themselves into human genomes randomly, our method is also much safer than using AAV2 and zinc-finger nuclease, as it causes little or no toxicity to the hES cells,” added Dr Wang. “We hope
that someday, cells derived from those iPS cells that are genetically modified with our
method could be used clinically for cancer therapy.”
Published recently in Nucleic Acids Research, IBN’s novel genetic engineering technique has great potential to advance the field of stem cell research, benefiting both basic science researchers and clinician scientists. The method developed by the IBN researchers allows repeated insertion of genes at the AAVS1 site, presenting the
opportunity to introduce a variety of therapeutic genes safely into the genome of hESCs and iPS cells for various medical applications. The modified hESCs can also be used as a starting material for generating transgenic hESCs for research, significantly reducing the amount of time spent on screening modified hESC clones.
“Using a new and better technology to treat chronic and degenerative diseases is the driving force behind our gene delivery research at IBN. Stem cell based therapy is one of our key research thrusts and we hope to unravel its potential to transform medical treatments with our innovative genetic modification approach,” added Professor Jackie.
Reference:
1. C. J. A. Ramachandra, M. Shahbazi, T. W. X. Kwang, Y. Choudhury, X. Y. Bak, J. Yang and S. Wang, “Efficient Recombinase-Mediated Cassette Exchange at the AAVS1 Locus in Human Embryonic Stem Cells Using Baculoviral Vectors,” Nucleic Acids Research, (2011) DOI: 10.1093/nar/gkr409.
For interviews, queries and photo requests, please contact:
Nidyah Sani
Phone: 65 6824 7005
Email: [email protected]
Elena Tan
Phone: 65 6824 7032
Email: [email protected]
About the Institute of Bioengineering and Nanotechnology
The Institute of Bioengineering and Nanotechnology (IBN) was established in 2003 and is spearheaded by its Executive Director, Professor Jackie Yi-Ru Ying, who has been on the Massachusetts Institute of Technology’s Chemical Engineering faculty since 1992, and was among the youngest to be promoted to Professor in 2001.
In 2008, Professor Ying was recognized as one of “One Hundred Engineers of the Modern Era” by the American Institute of Chemical Engineers for her groundbreaking work on nanostructured systems, nanoporous materials and host matrices for quantum dots and wires.
Under her direction, IBN conducts research at the cutting-edge of bioengineering and nanotechnology. Its programs are geared towards linking multiple disciplines across engineering, science and medicine to produce research breakthroughs that will improve healthcare and our quality of life.
IBN’s research activities are focused in the following areas:
• Drug and Gene Delivery, where the controlled release of therapeutics involve the use of functionalized polymers, hydrogels and biologics for targeting diseased cells and organs, and for responding to specific biological stimuli.
• Cell and Tissue Engineering, where biomimicking materials, stem cell technology, microfluidic systems and bioimaging tools are combined to develop novel approaches to regenerative medicine and artificial organs.
• Biodevices and Diagnostics, which involve nanotechnology and microfabricated platforms for high-throughput biomarker and drug screening, automated biologics synthesis, and rapid disease diagnosis.
• Pharmaceuticals Synthesis and Green Chemistry, which encompasses the efficient catalytic synthesis of chiral pharmaceuticals, and new nanocomposite materials for sustainable technology and alternative energy generation.
IBN's innovative research is aimed at creating new knowledge and intellectual properties in the emerging fields of bioengineering and nanotechnology to attract top-notch researchers and business partners to Singapore. Since 2003, IBN researchers have published over 670 papers in leading journals.
IBN also plays an active role in technology transfer and spinning off companies, linking the research institute and industrial partners to other global institutions. The Institute has a portfolio of over 729 patents/patent applications on its inventions, and welcomes industrial partners to collaborate on and co-develop its technologies. IBN has successfully commercialized 45 patents/patent applications.
IBN's current staff strength stands at over 170 scientists, engineers and medical doctors. With its multinational and multidisciplinary research staff, the institute is geared towards generating new biomaterials, devices, systems and processes to boost Singapore’s economy in the medical technology, pharmaceuticals, chemicals, consumer products and clean technology sectors.
IBN is also committed to nurturing young talents. Besides the training of PhD students, IBN has a Youth Research Program (YRP) for students and teachers from secondary schools, junior colleges, polytechnics, and universities. Since its inception in October 2003, IBN’s YRP has reached out to more than 44,000 students and teachers from 265 local and overseas schools and institutions.
For more information, please log on to: www.ibn.a-star.edu.sg.
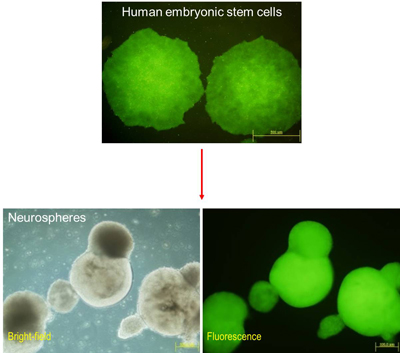
Figure 1: Genetically modified human embryonic stem cells (hESCs) and derived neurospheres. Using IBN’s BV-RMCE technique, a transgene (EGFP) was inserted into the AAVS1 locus on chromosome 19 in hESCs. Long-term expression of this gene was obtained in both hESCs and derived cell progenies. Cells derived from genetically modified hESCs can be used for transplantation in cell-based therapy.