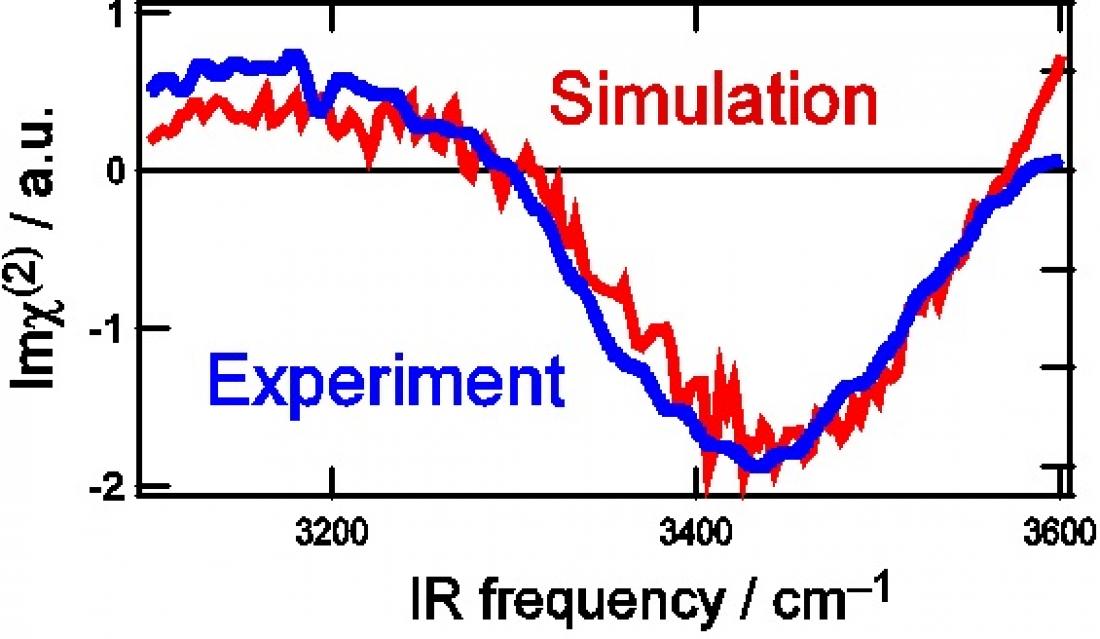
Imaginary part of 2nd order nonlinear susceptibility (Imχ(2)) spectra of water surfaces: (a) Experimental HOD (blue line), (b) simulated HOD (red). The concentration of isotopes is H2O : HOD : D2O = 1: 6: 9.
Findings by researchers at the RIKEN Advanced Science Institute and their colleagues at Tohoku University and in the Netherlands have resolved a long-standing debate over the structure of water molecules at the water surface. Published in the Journal of The American Chemical Society, the research combines theoretical and experimental techniques to pinpoint, for the first time, the origin of water’s unique surface properties in the interaction of water pairs at the air-water interface.
The most abundant compound on the Earth’s surface, water is essential to life and has shaped the course of human civilization. As perhaps the most common liquid interface, the air-water interface offers insights into the surface properties of water in everything from atmospheric and environmental chemistry, to cellular biology, to regenerative medicine. Yet despite its ubiquity, the structure of this interface has remained shrouded in mystery.
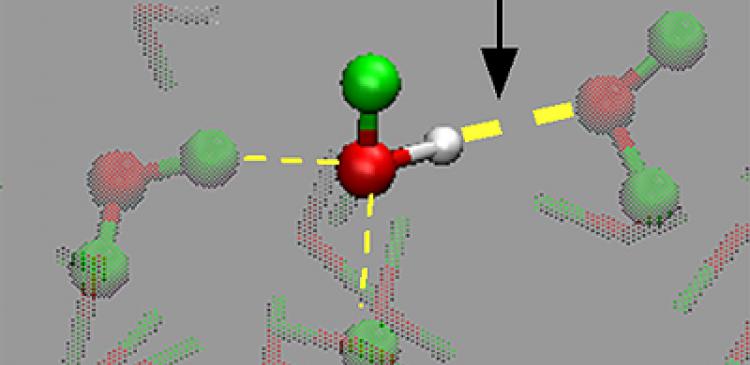
At the heart of this mystery are two broad bands in the vibrational spectrum for surface water resembling those of bulk ice and liquid water. Whether these bands are the result of hydrogen bonds themselves, of intra-molecular coupling between hydrogen bonds within a single water molecule, or of inter-molecular coupling between adjacent water molecules, is a source of heated debate. One popular but controversial hypothesis suggests one of the spectral bands corresponds to water forming an actual tetrahedral “ice-like” structure at the surface, but this interpretation raises issues of its own.
The researchers set out to resolve this debate through a comprehensive study combining theory and experiment. For their experiments, they applied a powerful spectroscopy technique developed at RIKEN to selectively pick out surface molecules and rapidly measure their spectra. To eliminate coupling effects, which are difficult to reproduce in simulations, they used water diluted with D2O (heavy water) and HOD (water with one hydrogen atom, H, replaced by deuterium, D). Doing so eliminates coupling of OH bonds within a single molecule (since there is only one OH bond) and reduces the overall concentration of OH bonds in the solution, suppressing intermolecular coupling.
With other influences removed, the researchers at last pinpointed the source of water’s unique surface structure not in an “ice-like” structure, but in the strong hydrogen bonding between water pairs at the outermost surface. The extremely good match between experimental and theoretical results confirms this conclusion, at long last bringing clarity to the debate over the structure of the water surface and setting the groundwork for fundamental advances in a range of scientific fields.
For more information, please contact:
Tahei Tahara
Satoshi Nihonyanagi
Molecular Spectroscopy Laboratory
RIKEN Advanced Science Institute
Tel: +81-(0)48-467-7928 / Fax: +81-(0)48-467-4539
Global Relations Office
RIKEN
Tel: +81-(0)48-462-1225 / Fax: +81-(0)48-463-3687
Email: [email protected]
Reach us on Twitter: @rikenresearch
Reference
Satoshi Nihonyanagi, Tatsuya Ishiyama, Touk-kwan Lee, Shoichi Yamaguchi, Mischa Bonn , Akihiro Morita and Tahei Tahara. "Unified Molecular View of Air/Water Interface Based on Experimental and Theoretical (2) Spectra of Isotopically Diluted Water Surface." Journal of The American Chemical Society, 2011, DOI: 10.1021/ja2053754
About RIKEN
RIKEN is Japan’s flagship research institute devoted to basic and applied research. Over 2500 papers by RIKEN researchers are published every year in reputable scientific and technical journals, covering topics ranging across a broad spectrum of disciplines including physics, chemistry, biology, medical science and engineering. RIKEN’s advanced research environment and strong emphasis on interdisciplinary collaboration has earned itself an unparalleled reputation for scientific excellence in Japan and around the world.
About the Advanced Science Institute
The RIKEN Advanced Science Institute (ASI) is an interdisciplinary research institute devoted to fostering creative, curiosity-driven basic research and sowing the seeds for innovative new projects. With more than 700 full-time researchers, the ASI acts as RIKEN’s research core, supporting inter-institutional and international collaboration and integrating diverse scientific fields including physics, chemistry, engineering, biology and medical science.