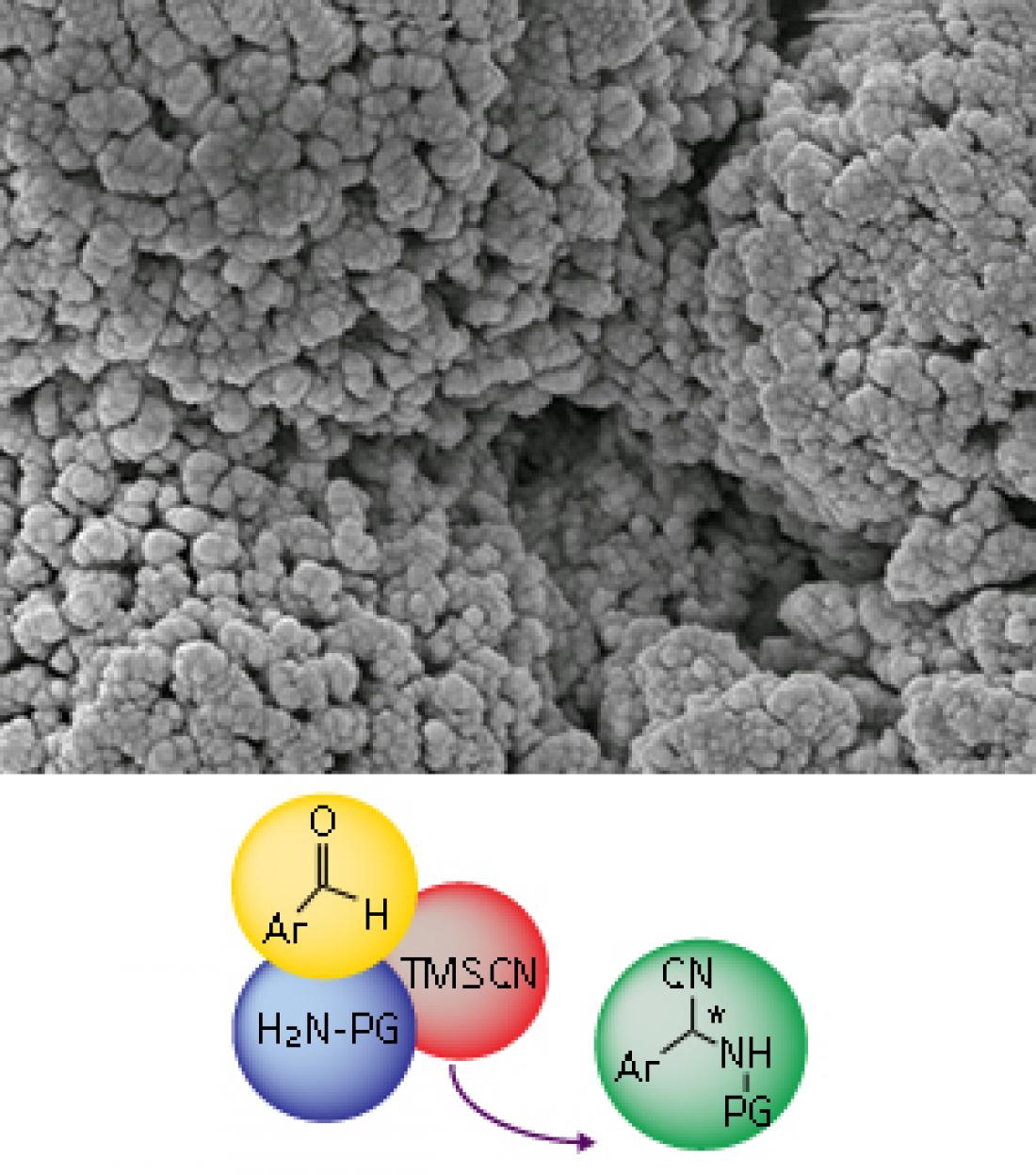
The self-supporting catalyst (top) is covered with tiny chiral pockets, within which simple starting materials (bottom, left) are converted into chiral amino acid precursors (bottom, right). © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (top image)
Cyanide exposure can be lethal, but with careful handling, the molecule can be a very useful chemical building block. For example, from cyanide chemists can make life-essential amino acids that are in great demand as food additives and components in pharmaceutical production. The key step in this process is called the asymmetric Strecker reaction. Until now, this reaction has needed complex and costly catalysts, restricting its use to small-scale laboratory research. A new Strecker catalyst more amenable to scale-up is now available, thanks to Abdul Seayad, Balamurugan Ramalingam and their co-workers at the A*STAR Institute of Chemical and Engineering Sciences in Singapore. The catalyst also offers a safer way to handle the cyanide.
Seayad and Ramalingam made their Strecker catalyst from an inexpensive material based on titanium. It is called the self-supported chiral titanium cluster (SCTC). When warmed in the presence of water, the SCTC precursor assembles into robust solid clusters (see image). The key to its performance in the Strecker reaction is that the surface of each cluster is covered in tiny asymmetric “chiral pockets”, says Seayad. The reactions take place in these pockets, generating molecules that are a trivial chemical step away from amino acids.
Amino acids are chiral: they can exist in either of two mirror-image forms called enantiomers. For many applications — such as pharmaceutical production — chemists need a pure supply of only one enantiomer. Seayad and Ramalingam found that SCTC’s chiral pockets very selectively produce one enantiomer over the other, with a purity — or ‘enantiomeric excess’ — of up to 99%. Unlike previous catalysts, which required temperatures as low as -30 °C to operate effectively, the researchers achieved this selectivity at room temperature.
Stability is a further advantage of SCTC. The catalyst is impervious to air or moisture, and remains stable to 300 °C, making it well suited to use in a continuous flow reactor. The researchers could pack the catalyst into a cartridge and pump through the cyanide and other starting materials, generating amino acids in a steady stream. Safety is another key advantage, says Ramalingam. “Since only a limited amount of cyanide is present at the reaction zone at any point in time, any unforeseen situation can be easily handled,” he explains.
So far, the researchers have used an expensive reagent called TMSCN as their cyanide source. They are currently researching ways to generate SCTC in situ from inexpensive salts. “We will also evaluate the feasibility of up-scaling the reaction under flow conditions,” Seayad says.
---
The A*STAR-affiliated researchers contributing to this research are from the Institute of Chemical and Engineering Sciences
References
Seayad, A. M., Ramalingam, B., Chai, C. L. L., Li, C., Garland, M. V. & Yoshinaga, K. Self-supported chiral titanium cluster (SCTC) as a robust catalyst for the asymmetric cyanation of imines under batch and continuous flow at room temperature. Chemistry – A European Journal 18, 5693–5700 (2012).