Researchers have discovered that the the age of the mice impacts microRNAs.
A recent study has reported that changes in mice sperm microRNAs brought about by aging may affect the growth and development of offspring. The finding adds to the growing literature on the effects of paternal aging on offspring.
Details of the study were published in the journal Scientific Reports on December 7, 2023.
Marriages and childbearing later in life are increasingly becoming the norm. Whilst the impacts of maternal age on offspring, such as a higher risk of miscarriage and Down syndrome, are widely understood, the impacts from the paternal side are less so.
Yet this is changing. Recent epidemiological studies have demonstrated that paternal aging exerts a more substantial influence on the heightened risk of neurodevelopmental disorders such as autism spectrum disorder.
A research team led by Professor Noriko Osumi from the Department of Developmental Neuroscience at the Tohoku University Graduate School of Medicine has previously revealed that epigenetic factors, including histone modifications in spermatogenesis and DNA methylation in mice sperm, undergo changes with age. These alterations might lead to transgenerational effects.
However, the impact of paternal aging on microRNAs (miRNAs), small, non-coding RNA molecules that play a crucial role in regulating gene expression, remains under explored.
To rectify this, the same research team has conducted a comprehensive analysis of age-related variations in microRNAs in mice sperm. They compared microRNAs in sperm from mice aged 3, 12, and 20 months and identified the microRNAs that had changed in quantity.
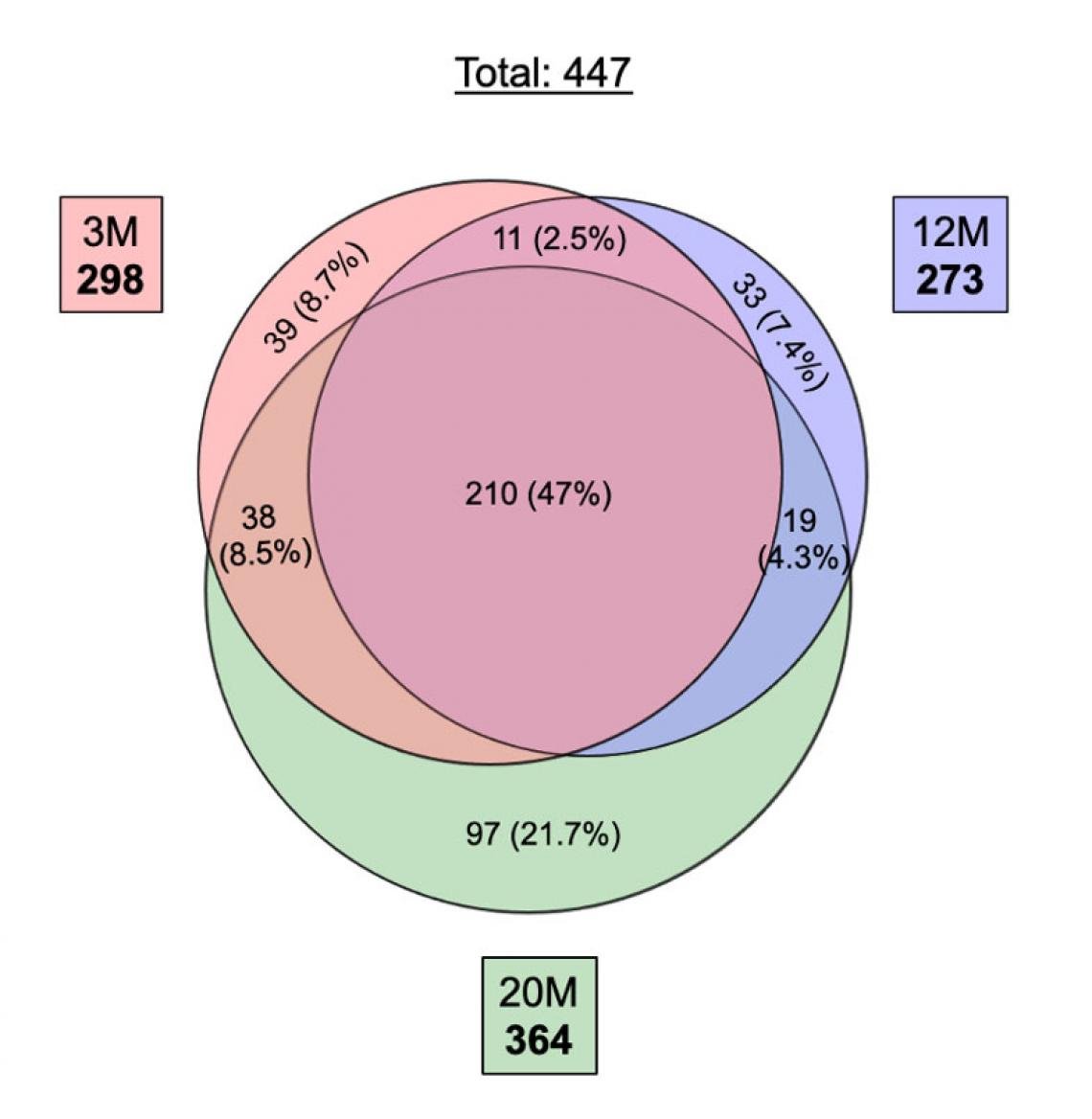
Venn diagram showing the number of miRNAs expressed in sperm samples from mice aged 3 M, 12 M and 20 M. A total of 447 miRNAs were expressed, and about half of them are common.
The researchers discovered significant age-associated differences in the microRNAs. Some changes were in microRNAs responsible for regulating the nervous system and genes related to autism spectrum disorder, and these altered microRNAs included those transferred to fertilized eggs.
"Our study reveals the potential association between alteration in sperm microRNAs caused by paternal aging, underscoring the significance of investigating the impact of sperm microRNAs on offspring, an aspect that has been relatively overlooked in previous research," states Osumi.
The anticipation is that further exploration of epigenetic factors, specifically microRNAs, will not only contribute to unraveling the pathogenic mechanisms underlying neurodevelopmental disorders but will also offer insights into promoting the health and disease prevention of successive generations.
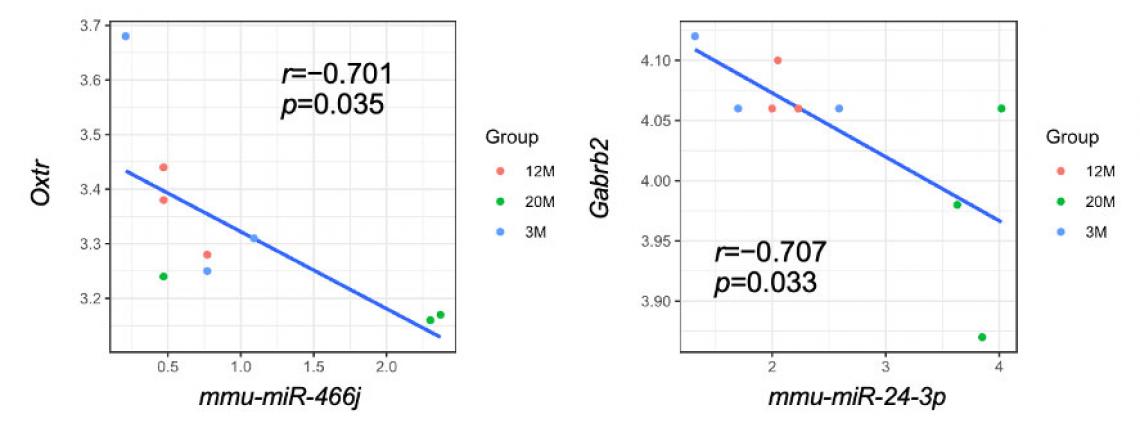
Correlation analyses between genes associated with ASD whose expression levels varied between groups and their regulatory miRNAs. Two genes important for brain function, Oxtr and Gabrb2, are regulated by miR-466j (left) and miR-24-3p (right), microRNAs whose expression levels change with aging.
Osumi points out that their study widens the net when it comes to exploring the link between paternal age and potential health complications in children. "While the age-related changes in oocytes are well-documented, the focus has predominantly centered on the fertility of sperm. Recognizing the myriad epigenetic transformations associated with sperm aging, as exemplified by the microRNAs examined in this study, becomes imperative."
The findings also gain relevance in the context of Japan's rapidly declining birthrate, which necessitates incorporating the perspective on sperm-related factors in advancing reproductive medicine.
The expression levels of miR-10a-5p (left) and miR-146a-5p (right), microRNAs reported to be transferred to the fertilized egg, changes with age.